Gynosampler
GUDID 05425017500377
The Gynosampler is an endometrial suction curette for a histologic lining biopsy.
Gynetics Medical Products NV
Endometrial biopsy kit| Primary Device ID | 05425017500377 |
| NIH Device Record Key | c96be6b0-7813-4653-b746-1c139e6f4b25 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Gynosampler |
| Version Model Number | #9164 |
| Company DUNS | 283088573 |
| Company Name | Gynetics Medical Products NV |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +3211645872 |
| info@gynetics.be |
Device Dimensions
| Outer Diameter | 2.93 Millimeter |
| Length | 262 Millimeter |
Operating and Storage Conditions
| Storage Environment Temperature | Between 0 Degrees Celsius and 30 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 05425017500377 [Primary] |
| GS1 | 15425017500374 [Package] Package: [25 Units] In Commercial Distribution |
| GS1 | 75425017500376 [Package] Package: [250 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K903002
FDA Product Code
| HFF | Aspirator, Endometrial |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | true |
[05425017500377]
Ethylene Oxide
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-10-04 |
Devices Manufactured by Gynetics Medical Products NV
| 05425017501022 - Embryo transfer catheter | 2020-02-19 Embryo transfer catheter used for introduction of embryos into the uterine cavity. |
| 25425017502009 - Intrauterine insemination cannula | 2019-01-23 Intra uterine Artificial Insemination |
| 25425017502016 - Intrauterine insemination cannula | 2019-01-23 Intra uterine Artificial Insemination |
| 25425017502023 - Intrauterine insemination cannula | 2019-01-23 Intrauterine insemination cannula |
| 25425017502030 - Intrauterine insemination cannula | 2019-01-23 Intrauterine insemination cannula |
| 25425017502047 - Intrauterine insemination cannula | 2019-01-23 Intrauterine insemination cannula |
| 25425017502054 - Intrauterine insemination cannula | 2019-01-23 Intrauterine insemination cannula |
| 25425017502061 - Intrauterine insemination cannula | 2019-01-23 Intrauterine insemination cannula |
Trademark Results [Gynosampler]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
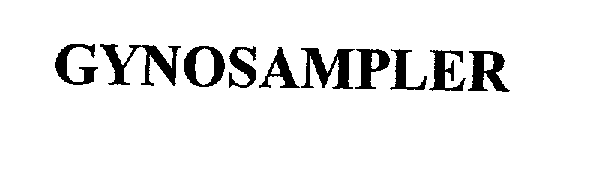 GYNOSAMPLER 75848837 2424958 Live/Registered |
GYNETICS HOLDING BVBA 1999-11-15 |
 GYNOSAMPLER 74155328 1774652 Dead/Cancelled |
GynoPharma, Inc. 1991-04-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.