Lifehood
GUDID 06932825301779
Blood Pressure Monitor
Guangdong Transtek Medical Electronics Co.,Ltd
Automatic-inflation electronic sphygmomanometer, portable, arm/wrist| Primary Device ID | 06932825301779 |
| NIH Device Record Key | a1e5af3f-6027-4b2d-9114-63a781a9cce5 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Lifehood |
| Version Model Number | TMB-2080-K |
| Company DUNS | 679794078 |
| Company Name | Guangdong Transtek Medical Electronics Co.,Ltd |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Unsafe |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 06932825301779 [Primary] |
FDA Product Code
| DXN | System, Measurement, Blood-Pressure, Non-Invasive |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-08-30 |
| Device Publish Date | 2023-08-22 |
On-Brand Devices [Lifehood]
| 26932825317750 | TMB-1775 |
| 06932825301779 | Blood Pressure Monitor |
| 06932825301472 | Blood pressure monitor:TMB-1491-S-072 |
| 06932825301465 | Blood pressure monitor:TMB-2085-008 |
| 06932825301458 | Blood pressure monitor:TMB-2084-KT-003 |
Trademark Results [Lifehood]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LIFEHOOD 88460611 5937722 Live/Registered |
Guangdong Transtek Medical Electronics Co., Ltd. 2019-06-05 |
 LIFEHOOD 87955879 not registered Live/Pending |
GUANGDONG TRANSTEK MEDICAL ELECTRONICS CO., LTD. 2018-06-10 |
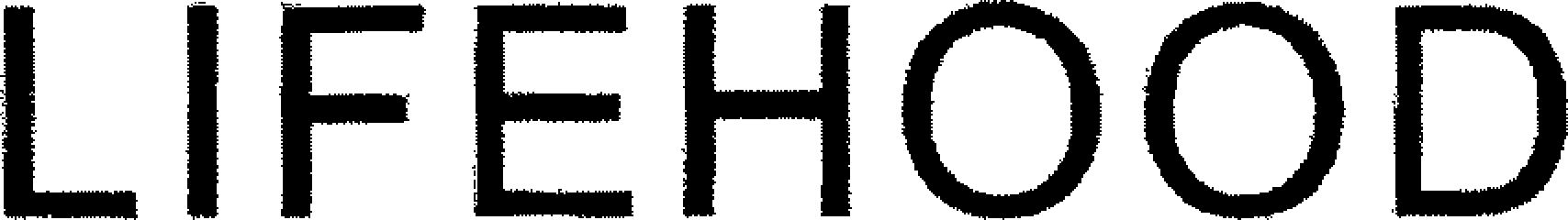 LIFEHOOD 79244379 not registered Live/Pending |
Guangdong Transtek Medical Electronics Co., Ltd. 2018-07-26 |
 LIFEHOOD 74619900 2021313 Dead/Cancelled |
Knight International Holdings, Inc. 1995-01-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.