Double Dove 0200
GUDID 06934755702008
Sterile hypodermic syringe for single use, with needle, is intended to be used for medical purposes to inject fluid into or withdraw fluid from the body. Volume: 1mL; Needle size: 0.45*12.7mm RWLB; Nozzle type: Luer Lock.
Jumin Bio-Technologies, Co., Ltd.
General-purpose syringe/needle| Primary Device ID | 06934755702008 |
| NIH Device Record Key | b01dcb5a-6407-4f88-8d2f-b6dcbe478c3b |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Double Dove |
| Version Model Number | 0200 |
| Catalog Number | 0200 |
| Company DUNS | 544382151 |
| Company Name | Jumin Bio-Technologies, Co., Ltd. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 06934755702008 [Primary] |
| GS1 | 16934755702005 [Package] Package: shelf carton [100 Units] In Commercial Distribution |
| GS1 | 26934755702002 [Package] Contains: 16934755702005 Package: case carton [30 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K090929
FDA Product Code
| FMF | Syringe, Piston |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-02-08 |
| Device Publish Date | 2021-01-29 |
On-Brand Devices [Double Dove]
| 16934755702425 | Sterile hypodermic syringe for single use, with needle, is intended to be used for medical purpo |
| 16934755702227 | Sterile hypodermic syringe for single use, with needle, is intended to be used for medical purpo |
| 06934755702060 | Sterile hypodermic syringe for single use, with needle, is intended to be used for medical purpo |
| 16934755702050 | Sterile hypodermic syringe for single use, with needle, is intended to be used for medical purpo |
| 16934755702043 | Sterile hypodermic syringe for single use, with needle, is intended to be used for medical purpo |
| 16934755702036 | Sterile hypodermic syringe for single use, with needle, is intended to be used for medical purpo |
| 06934755702022 | Sterile hypodermic syringe for single use, with needle, is intended to be used for medical purpo |
| 06934755702008 | Sterile hypodermic syringe for single use, with needle, is intended to be used for medical purpo |
Trademark Results [Double Dove]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
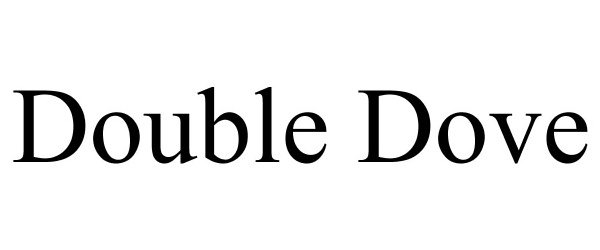 DOUBLE DOVE 77324654 not registered Dead/Abandoned |
Player Based Gaming, LLC. 2007-11-08 |
 DOUBLE DOVE 76626796 not registered Dead/Abandoned |
ABULHAJ, RAMZI 2005-01-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.