TRi-WAVE
GUDID 06942808291409
TENS stands for Transcutaneous Electrical Nerve Stimulation. The TRi-WAVE TENS system is used to provide symptomatic pain relief for chronic, acute or post-operative pain. EMS stands for Electrical Neuromuscular Stimulator. The TRi-WAVE EMS system is indicated for: • Relaxation of muscle spasm; • Increasing local blood circulation and muscle re-education; • Prevention or retardation of disuse atrophy; • Prevention of venous thrombosis of the calf muscles immediately after surgery; • Maintaining or increasing range of motion. IF stands for Interferential Stimulation. The TRi-WAVE IF system is indicated for: • Symptomatic relief of chronic intractable pain.
EasyMed Instruments Co., Ltd.
Physical therapy transcutaneous neuromuscular electrical stimulation system| Primary Device ID | 06942808291409 |
| NIH Device Record Key | 485c8c2c-3301-4e01-b70a-a3edab039db4 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | TRi-WAVE |
| Version Model Number | TRi-WAVE |
| Company DUNS | 526951168 |
| Company Name | EasyMed Instruments Co., Ltd. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 06942808291409 [Primary] |
FDA Product Code
| IPF | Stimulator, Muscle, Powered |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-11-23 |
| Device Publish Date | 2021-11-15 |
Devices Manufactured by EasyMed Instruments Co., Ltd.
| 06942808291478 - IF-4 MultiWave | 2024-01-29 EMS stands for Electrical Neuromuscular Stimulator. The IF-4 MultiWave EMS system is indicated for: • Relaxation of muscle spa |
| 06942808221055 - iTENS | 2024-01-26 iTENS is intended for the relief of pain associated with sore or aching muscles of the lower back, arms, or legs due to strain f |
| 06942808241015 - KGL-1400-F | 2021-11-25 KGL-1400-F is intended for the relief of pain associated with sore or aching muscles of the lower back, arms, or legs due to str |
| 06942808261013 - Ultima Combo | 2021-11-25 Ultima Combo is a Transcutaneous Electrical Nerve Stimulator which is used to provide symptomatic pain relief for chronic, acute |
| 06942808261020 - Relief Combo | 2021-11-25 Relief Combo is a Transcutaneous Electrical Nerve Stimulator which is used to provide symptomatic pain relief for chronic, acute |
| 06942808291027 - Parasym | 2021-11-25 Parasym is a Transcutaneous Electrical Nerve Stimulator, which is used to provide symptomatic pain relief for chronic, acute or |
| 06942808221031 - TENS U5 | 2021-11-23 TENS U5 is a Transcutaneous Electrical Nerve Stimulator which is used to provide symptomatic pain relief for chronic, acute or p |
| 06942808221048 - Ultima 20 | 2021-11-23 Ultima 20 is a Transcutaneous Electrical Nerve Stimulator which is used to provide symptomatic pain relief for chronic, acute or |
Trademark Results [TRi-WAVE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
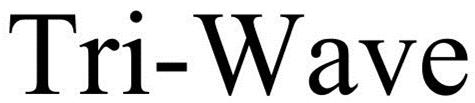 TRI-WAVE 86481791 4807201 Live/Registered |
ELITE MEDICAL SUPPLY OF NY, LLC 2014-12-16 |
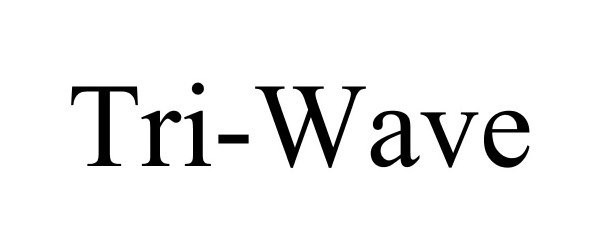 TRI-WAVE 78808267 not registered Dead/Abandoned |
Panlong International, Inc. 2006-02-06 |
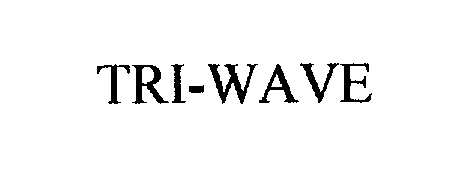 TRI-WAVE 76206665 2640720 Dead/Cancelled |
Frontier Medical, Inc. 2001-02-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.