babaka
GUDID 06950715503294
Back Brace
Cofoe Medical Technology Co.,Ltd
Lumbar spine orthosis| Primary Device ID | 06950715503294 |
| NIH Device Record Key | b8d044cc-750f-4f9a-b38c-0e7abd52bd03 |
| Commercial Distribution Discontinuation | 2030-04-27 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | babaka |
| Version Model Number | New E+ |
| Company DUNS | 544425204 |
| Company Name | Cofoe Medical Technology Co.,Ltd |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 06950715503294 [Primary] |
FDA Product Code
| IPT | Orthosis, Thoracic |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-07-22 |
| Device Publish Date | 2024-07-12 |
On-Brand Devices [babaka]
| 06950715503294 | Back Brace |
| 06950715503287 | Back Brace |
| 06950715503270 | Lumbar Support |
| 06950715504444 | Back Brace |
| 06950715504437 | Back Brace |
| 06950715504512 | Back Brace |
| 06950715504505 | Back Brace |
| 06950715505564 | Back Brace |
Trademark Results [babaka]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BABAKA 98710297 not registered Live/Pending |
Cofoe Medical Technology Co., Ltd. 2024-08-21 |
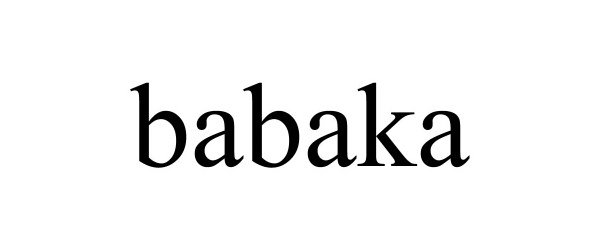 BABAKA 88698904 not registered Live/Pending |
Hangzhou Jessica E-commerce Co., Ltd. 2019-11-19 |
 BABAKA 88549220 not registered Live/Pending |
Han,Jinfeng 2019-07-30 |
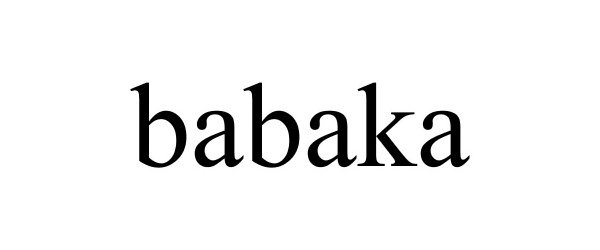 BABAKA 88347683 5868042 Live/Registered |
Hangzhou Jessica E-commerce Co., Ltd. 2019-03-20 |
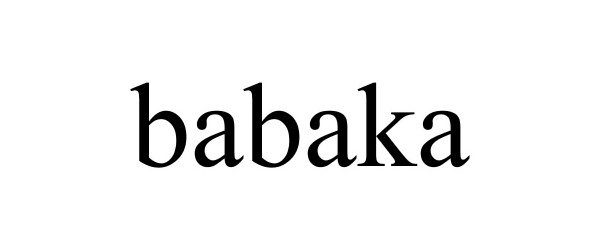 BABAKA 87715212 5518672 Live/Registered |
Hangzhou Jessica E-commerce Co., Ltd. 2017-12-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.