Equate
GUDID 06970742221428
Equate Pain Therapy This is intended for the relief of pain associated with sore or aching muscles in the following anatomical locations: the lower back, shoulders, arms and legs due to strain from exercise or ADL’s (activities of daily living) principally regular household and work activities.
Guangzhou Xinbo Electronic Co., Ltd.
Physical therapy transcutaneous neuromuscular electrical stimulation system| Primary Device ID | 06970742221428 |
| NIH Device Record Key | 9dff2326-91bd-4896-8cee-67fb16b74cd2 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Equate |
| Version Model Number | P.T.S-II |
| Company DUNS | 530683813 |
| Company Name | Guangzhou Xinbo Electronic Co., Ltd. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 06970742221428 [Primary] |
FDA Product Code
| NUH | Stimulator, Nerve, Transcutaneous, Over-The-Counter |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-03-09 |
| Device Publish Date | 2020-03-01 |
Devices Manufactured by Guangzhou Xinbo Electronic Co., Ltd.
| 06970742220339 - DR-HOS | 2025-07-08 XJ-F-30 |
| 06970742220278 - DR-HOS | 2023-11-20 Adjustable Pillow – King size |
| 06970742220285 - DR-HOS | 2023-11-20 Adjustable Pillow – Queen size |
| 06970742220292 - DR-HOS | 2023-11-20 Adjustable Pillow – Children size |
| 06970742220308 - DR-HOS | 2023-11-20 Adjustable Pillow – Travel size |
| 06970742220315 - DR-HOS | 2023-11-20 Adjustable Pillow – Leg |
| 06970742220001 - DR -HOS | 2022-03-09 Circulation Promoter Pro (CP-Pro) |
| 06970742220162 - DR -HOS | 2022-03-09 Back Belt with Electrodes (sticky gel type) |
Trademark Results [Equate]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
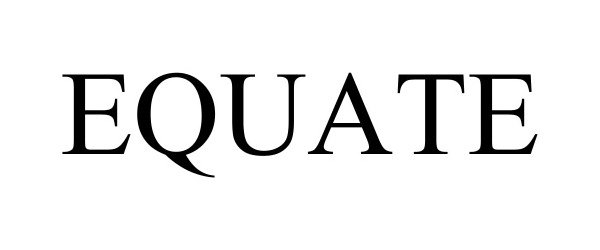 EQUATE 98371675 not registered Live/Pending |
Walmart Apollo, LLC 2024-01-23 |
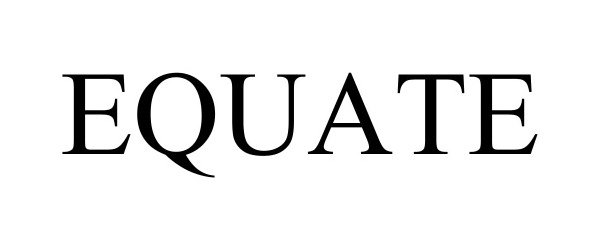 EQUATE 98246521 not registered Live/Pending |
BASF Corporation 2023-10-30 |
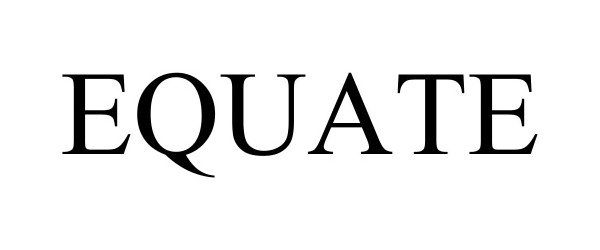 EQUATE 97936656 not registered Live/Pending |
Walmart Apollo, LLC 2023-05-15 |
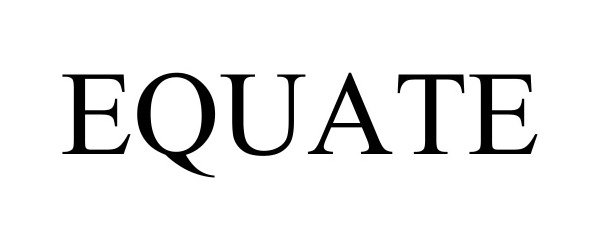 EQUATE 97551729 not registered Live/Pending |
Walmart Apollo, LLC 2022-08-17 |
 EQUATE 97471812 not registered Live/Pending |
Walmart Apollo, LLC 2022-06-23 |
 EQUATE 97303593 not registered Live/Pending |
Walmart Apollo, LLC 2022-03-09 |
 EQUATE 97303573 not registered Live/Pending |
Walmart Apollo, LLC 2022-03-09 |
 EQUATE 97096565 not registered Live/Pending |
Walmart Apollo, LLC 2021-10-28 |
 EQUATE 97081722 not registered Live/Pending |
Walmart Apollo, LLC 2021-10-19 |
 EQUATE 90045989 not registered Live/Pending |
Equate Petrochemical Company 2020-07-10 |
 EQUATE 88658120 not registered Live/Pending |
Walmart Apollo, LLC 2019-10-17 |
 EQUATE 88040498 not registered Live/Pending |
Walmart Apollo, LLC 2018-07-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.