EPULSE
GUDID 06971712727520
Hong Qiangxing (Shen Zhen) Electronics Limited
Physical therapy transcutaneous neuromuscular electrical stimulation system| Primary Device ID | 06971712727520 |
| NIH Device Record Key | d2de3eb4-5a24-4c23-a747-92f08a671575 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | EPULSE |
| Version Model Number | SM9187 |
| Company DUNS | 421286946 |
| Company Name | Hong Qiangxing (Shen Zhen) Electronics Limited |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 06971712727520 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K190009
FDA Product Code
| NGX | Stimulator, Muscle, Powered, For Muscle Conditioning |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-12-10 |
| Device Publish Date | 2024-12-02 |
On-Brand Devices [EPULSE]
| 06971712720040 | TENS |
| 06971712720026 | TENS |
| 06971712725304 | SM9079 |
| 06971712720033 | Mini Massager ULTRA 1420 |
| 06971712727520 | SM9187 |
Trademark Results [EPULSE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
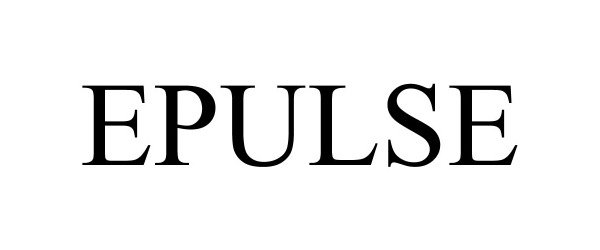 EPULSE 97097344 not registered Live/Pending |
Epulse LLC 2021-10-28 |
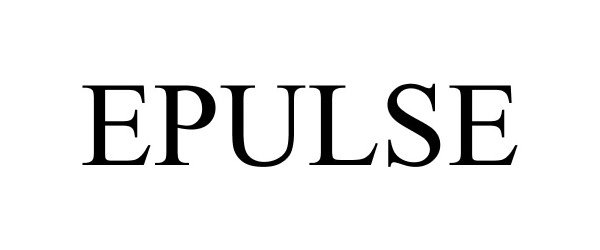 EPULSE 86529986 not registered Dead/Abandoned |
Pulse iQ, Inc. 2015-02-10 |
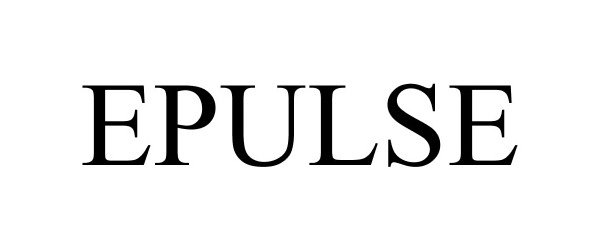 EPULSE 86515091 4865688 Live/Registered |
ePulse Limited 2015-01-27 |
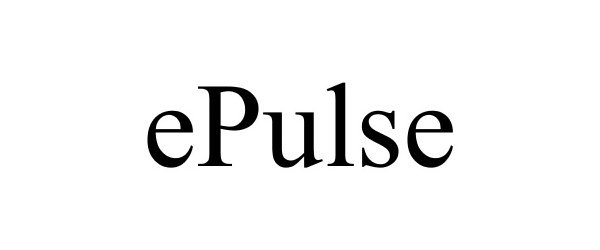 EPULSE 86097681 4676375 Live/Registered |
Mueller International, LLC 2013-10-22 |
 EPULSE 79018481 3209368 Dead/Cancelled |
ePULSE Limited 2004-10-12 |
 EPULSE 77747722 not registered Dead/Abandoned |
Enalasys, Inc. 2009-05-29 |
 EPULSE 77228085 3385180 Live/Registered |
Primordial Diagnostics, Inc. 2007-07-12 |
 EPULSE 77002672 3313246 Dead/Cancelled |
Impact Sports Technologies Inc. 2006-09-19 |
 EPULSE 76052070 not registered Dead/Abandoned |
InterX Technologies. Inc. 2000-05-19 |
 EPULSE 75271138 2149377 Dead/Cancelled |
MTS, Incorporated 1997-04-07 |
 EPULSE 74707808 1993203 Dead/Cancelled |
MTS, Incorporated 1995-07-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.