BIAL
GUDID 06971911291150
10ML LUER SLIP
Jiangsu Micsafe Medical Tehcnology Co.,LTD.
General-purpose syringe, single-use| Primary Device ID | 06971911291150 |
| NIH Device Record Key | c14d4536-06f2-467b-93b8-7581bc561e1b |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | BIAL |
| Version Model Number | 1 |
| Company DUNS | 554400604 |
| Company Name | Jiangsu Micsafe Medical Tehcnology Co.,LTD. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 06971911291150 [Primary] |
FDA Product Code
| FMF | Syringe, Piston |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-06-08 |
| Device Publish Date | 2023-05-31 |
On-Brand Devices [BIAL]
| 06971911291167 | 20ML LUER SLIP |
| 06971911291150 | 10ML LUER SLIP |
| 06971911291143 | 5ML LUER SLIP |
| 06971911291129 | 20ML LUER SLIP |
| 06971911291112 | 10ML LUER SLIP |
| 06971911291105 | 5ML LUER SLIP |
| 06971911291099 | 2ML LUER SLIP |
Trademark Results [BIAL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BIAL 87932884 5629813 Live/Registered |
BISO Co., Ltd. 2018-05-23 |
 BIAL 86376625 4811225 Live/Registered |
ShenZhen JinDanuo Information Technology Co.,Ltd. 2014-08-26 |
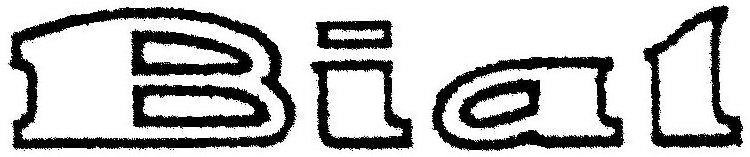 BIAL 85583401 4490162 Live/Registered |
BIAL-Portela & Ca., S.A. 2012-03-29 |
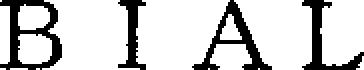 BIAL 79224808 not registered Dead/Abandoned |
BISO Co., Ltd. 2017-10-30 |
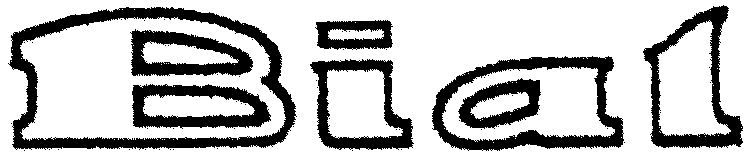 BIAL 79005898 3076871 Dead/Cancelled |
BIAL - PORTELA & Cª., S.A. 2004-09-01 |
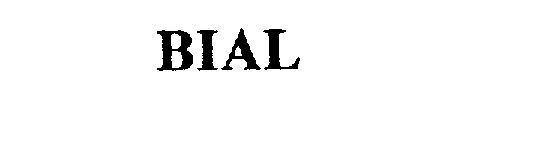 BIAL 75391671 2287116 Live/Registered |
MIBA GLEITLAGER GMBH 1997-11-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.