WiScope® 4202T0201W
GUDID 06972084140412
Image System
Ningbo Wise Optomech Technology Corporation
Endoscopic video image processing unit| Primary Device ID | 06972084140412 |
| NIH Device Record Key | 4f65d283-7caf-49d9-b327-5f7d86b2ab82 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | WiScope® |
| Version Model Number | OTU-T |
| Catalog Number | 4202T0201W |
| Company DUNS | 540094524 |
| Company Name | Ningbo Wise Optomech Technology Corporation |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 06972084140412 [Primary] |
FDA Product Code
| FAJ | Cystoscope And Accessories, Flexible/Rigid |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-01-22 |
| Device Publish Date | 2024-01-12 |
On-Brand Devices [WiScope®]
| 06972084140054 | Image System |
| 16972084140044 | Single-Use Digital Flexible Ureteroscope |
| 16972084140037 | Single-Use Digital Flexible Ureteroscope |
| 16972084140020 | Single-Use Digital Flexible Ureteroscope |
| 06972084140016 | Single-Use Digital Flexible Ureteroscope |
| 16972084140105 | Single-Use Digital Flexible UroScope |
| 16972084140099 | Single-Use Digital Flexible UroScope |
| 06972084140085 | Single-Use Digital Flexible UroScope |
| 16972084140075 | Single-Use Digital Flexible UroScope |
| 06972084140061 | Image System, adapting to a monitor of 16:9 aspect ratio |
| 06972084140399 | Image System, adapting to a monitor of 16:9 aspect ratio |
| 06972084140412 | Image System |
| 06972084140405 | Image System |
Trademark Results [WiScope]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
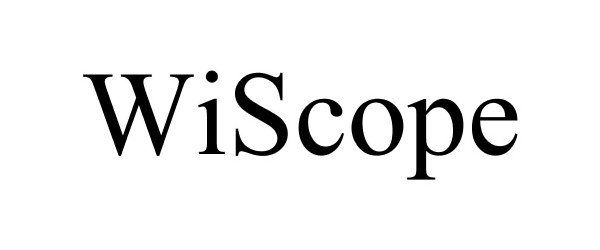 WISCOPE 88407180 not registered Live/Pending |
Inspectron, Inc. 2019-04-29 |
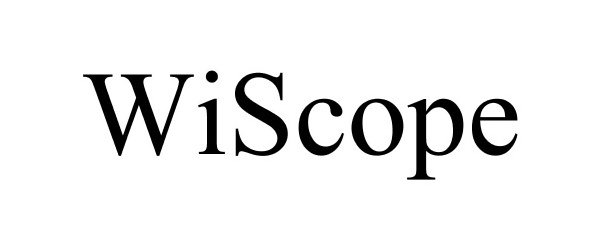 WISCOPE 87636989 5571987 Live/Registered |
OTU MEDICAL INC. 2017-10-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.