femometer
GUDID 06972412611836
Test for luteinizing hormone in urine to predict ovulation date, including meter and test sticks.
Hangzhou Laihe Biotech Co.,Ltd.
Luteinizing hormone (LH) IVD, kit, rapid ICT, self-testing| Primary Device ID | 06972412611836 |
| NIH Device Record Key | 77eff209-9d9a-48eb-9f66-2302d290d420 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | femometer |
| Version Model Number | FM-IVY-103 |
| Company DUNS | 543330912 |
| Company Name | Hangzhou Laihe Biotech Co.,Ltd. |
| Device Count | 20 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 06972412611836 [Primary] |
| GS1 | 06972412617098 [Unit of Use] |
FDA Product Code
| NGE | Test, Luteinizing Hormone (Lh), Over The Counter |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-06-06 |
| Device Publish Date | 2023-05-29 |
On-Brand Devices [ femometer]
| 06972412611843 | Test for luteinizing hormone in urine to predict ovulation date, test sticks refill pack. |
| 06972412611836 | Test for luteinizing hormone in urine to predict ovulation date, including meter and test sticks |
| 06972412612017 | This product is a rapid, one-step, visual test for the qualitative detection of follicle stimula |
| 06972412612000 | This product is a rapid, one-step, visual test for the qualitative detection of follicle stimula |
Trademark Results [femometer]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
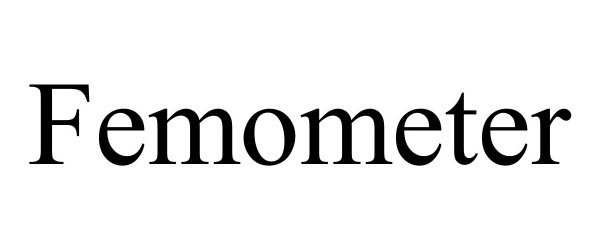 FEMOMETER 97455113 not registered Live/Pending |
Lingshu Technology (Hangzhou) Co., Ltd. 2022-06-13 |
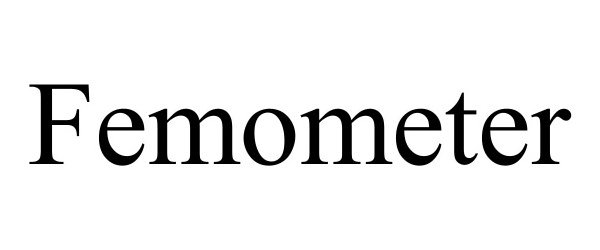 FEMOMETER 97455112 not registered Live/Pending |
Lingshu Technology (Hangzhou) Co., Ltd. 2022-06-13 |
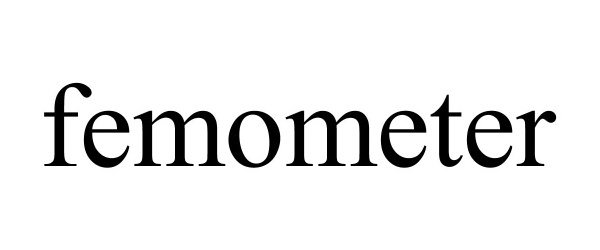 FEMOMETER 90368041 not registered Live/Pending |
HANGZHOU BANGTANG NETWORK TECHNOLOGY CO., LTD. 2020-12-08 |
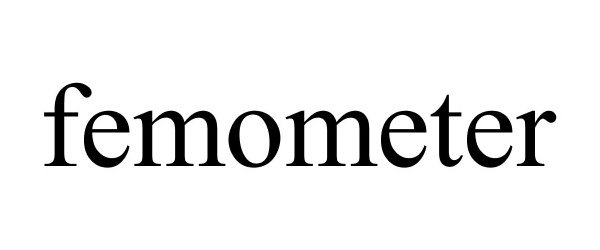 FEMOMETER 88447685 5926596 Live/Registered |
Hangzhou Bangtang Network Technology Co., Ltd. 2019-05-27 |
 FEMOMETER 86738179 5028466 Live/Registered |
HANGZHOU BANGTANG NETWORK TECHNOLOGY CO., LTD 2015-08-26 |
 FEMOMETER 86738159 not registered Dead/Abandoned |
Li, Xue 2015-08-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.