ZICON
GUDID 06974665662962
Yongkang Youha Electric Appliance Co.,Ltd
Electric-motor-driven wheelchair, occupant-controlled| Primary Device ID | 06974665662962 |
| NIH Device Record Key | 7651705f-9fbe-40be-a8ff-508e390fb5c3 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | ZICON |
| Version Model Number | YHWL002 |
| Company DUNS | 541069715 |
| Company Name | Yongkang Youha Electric Appliance Co.,Ltd |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 06974665662962 [Primary] |
FDA Product Code
| ITI | Wheelchair, Powered |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2025-03-07 |
| Device Publish Date | 2025-02-27 |
Devices Manufactured by Yongkang Youha Electric Appliance Co.,Ltd
| 06974665669756 - Juswell | 2026-02-03 |
| 06974665669688 - ESHINE | 2026-01-05 |
| 06974665669374 - Meo Move | 2025-12-25 |
| 06974665669442 - Meo Move | 2025-12-25 |
| 06974665669510 - FRITTON | 2025-12-25 |
| 06974665669060 - Zygenair | 2025-12-23 |
| 06974665669138 - Zygenair | 2025-12-23 |
| 06974665668902 - Neo Move | 2025-12-22 |
Trademark Results [ZICON]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZICON 97427101 not registered Live/Pending |
Sichuan Zejiang International Trade Co., Ltd 2022-05-25 |
 ZICON 90040858 not registered Live/Pending |
xiamen zicon Electronic Commerce Company Limited 2020-07-08 |
 ZICON 78250177 not registered Dead/Abandoned |
Zicon Ltd. 2003-05-15 |
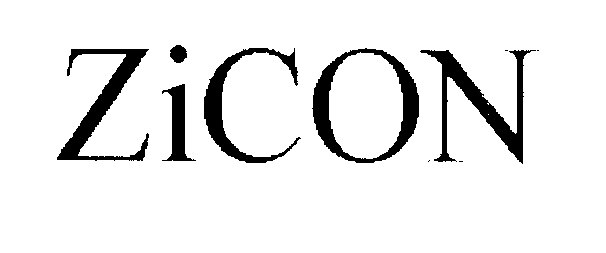 ZICON 78145187 2896327 Dead/Cancelled |
ZIPTRONIX, INC. 2002-07-18 |
 ZICON 74675788 1994997 Dead/Cancelled |
U.S. DESIGN, INC. 1995-05-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.