Equate
GUDID 07290006558145
PEPTONIC MEDICAL ISRAEL LTD
Vaginal pH screening IVD, kit, rapid colorimetric, clinical| Primary Device ID | 07290006558145 |
| NIH Device Record Key | 581c5374-2c37-414c-9134-bd5a24a19c33 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Equate |
| Version Model Number | F-1-VO-WAL02 |
| Company DUNS | 600850111 |
| Company Name | PEPTONIC MEDICAL ISRAEL LTD |
| Device Count | 2 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 07290006558145 [Unit of Use] |
| GS1 | 17290006558142 [Primary] |
FDA Product Code
| CEN | Dye-Indicator, Ph (Urinary, Non-Quantitative) |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-07-06 |
| Device Publish Date | 2023-06-28 |
Devices Manufactured by PEPTONIC MEDICAL ISRAEL LTD
| 07290006558091 - VagiVital VS | 2023-07-06 |
| 07290006558107 - Vagi-Screen Vaginal Health Test | 2023-07-06 |
| 07290006558114 - Florisense Vaginal Health Test | 2023-07-06 |
| 07290006558121 - Florisense Preconception Vaginal Health Test | 2023-07-06 |
| 07290006558138 - Feminine Screening Kit | 2023-07-06 |
| 07290006558145 - Equate | 2023-07-06 |
| 07290006558145 - Equate | 2023-07-06 |
| 07290006558152 - Clinistix Vaginal Health Test | 2023-07-06 |
| 07290006558169 - VS-SENSE | 2023-07-06 |
Trademark Results [Equate]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EQUATE 98371675 not registered Live/Pending |
Walmart Apollo, LLC 2024-01-23 |
 EQUATE 98246521 not registered Live/Pending |
BASF Corporation 2023-10-30 |
 EQUATE 97936656 not registered Live/Pending |
Walmart Apollo, LLC 2023-05-15 |
 EQUATE 97551729 not registered Live/Pending |
Walmart Apollo, LLC 2022-08-17 |
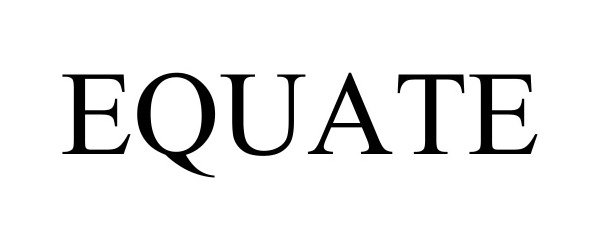 EQUATE 97471812 not registered Live/Pending |
Walmart Apollo, LLC 2022-06-23 |
 EQUATE 97303593 not registered Live/Pending |
Walmart Apollo, LLC 2022-03-09 |
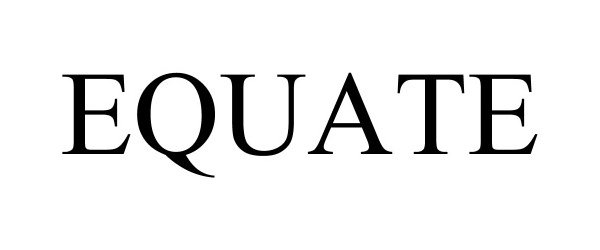 EQUATE 97303573 not registered Live/Pending |
Walmart Apollo, LLC 2022-03-09 |
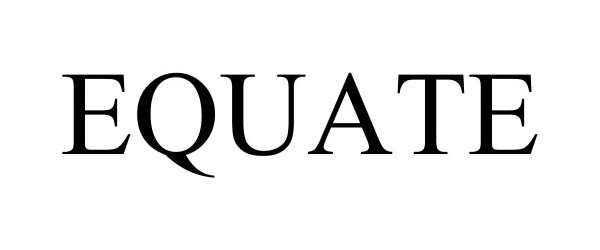 EQUATE 97096565 not registered Live/Pending |
Walmart Apollo, LLC 2021-10-28 |
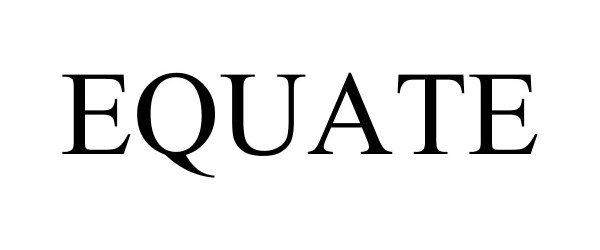 EQUATE 97081722 not registered Live/Pending |
Walmart Apollo, LLC 2021-10-19 |
 EQUATE 90045989 not registered Live/Pending |
Equate Petrochemical Company 2020-07-10 |
 EQUATE 88658120 not registered Live/Pending |
Walmart Apollo, LLC 2019-10-17 |
 EQUATE 88040498 not registered Live/Pending |
Walmart Apollo, LLC 2018-07-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.