Trolley MM60000-US
GUDID 07290016746730
EnerJet Trolley
VIORA LTD
Needleless medication/vaccine injector, pneumatic| Primary Device ID | 07290016746730 |
| NIH Device Record Key | 8776a559-e76f-4a6c-9560-0eaf5d3f4018 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Trolley |
| Version Model Number | MM60000-US |
| Catalog Number | MM60000-US |
| Company DUNS | 514612758 |
| Company Name | VIORA LTD |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 07290016746730 [Primary] |
FDA Product Code
| KZE | Injector, Fluid, Non-Electrically Powered |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2025-07-31 |
| Device Publish Date | 2025-05-22 |
Devices Manufactured by VIORA LTD
| 07290016746563 - V-IPL Optic set Full 6 filters | 2025-12-30 |
| 07290016746464 - V-ST Single Use tip | 2025-09-09 V-ST Single Use tip |
| 07290016746471 - V-VR Handpiece | 2025-09-09 V-VR Handpiece |
| 17290016746652 - V-FC Tip | 2025-09-09 V-FC Tip |
| 07290016746785 - V-FC Pro Full | 2025-09-09 Kit of V-FC Pro Handpiece, V-FC Pro Tips and maintenance kit |
| 07290016746808 - V-FC Pro Tip | 2025-09-09 |
| 07290016746815 - V-FC Pro Handpiece | 2025-09-09 |
| 07290016746716 - Console | 2025-07-31 EnerJet Console |
Trademark Results [Trolley]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TROLLEY 98880042 not registered Live/Pending |
Trolley Technologies Inc. 2024-12-02 |
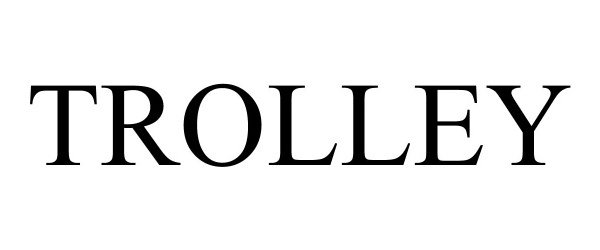 TROLLEY 97096864 not registered Live/Pending |
Trolley Technologies Inc. 2021-10-28 |
 TROLLEY 90261989 not registered Live/Pending |
Bismuth Media, LLC 2020-10-18 |
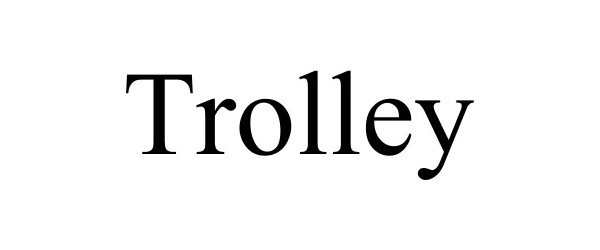 TROLLEY 90109693 not registered Live/Pending |
Bismuth Media, LLC 2020-08-12 |
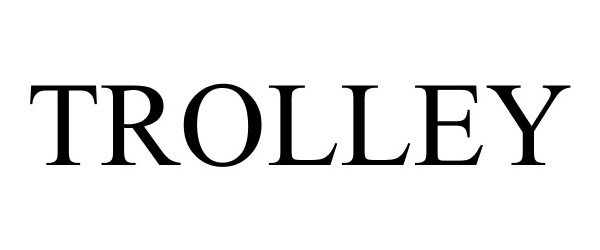 TROLLEY 88173263 not registered Live/Pending |
Coulter Ventures, LLC 2018-10-29 |
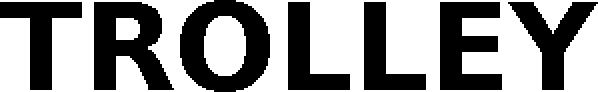 TROLLEY 79305158 not registered Live/Pending |
Systec & Solutions GmbH 2020-11-19 |
 TROLLEY 77414035 not registered Dead/Abandoned |
Tampa Sweethearts Cigar Company 2008-03-05 |
 TROLLEY 73534040 not registered Dead/Abandoned |
WOLVERINE WORLD WIDE, INC. 1985-04-25 |
 TROLLEY 73306004 1198874 Dead/Cancelled |
Old Post Yarn Works Ltd. 1981-04-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.