AcuPulse™ DUO RG-1000000
GUDID 07290109146027
LUMENIS LTD.
General/multiple surgical solid-state/carbon dioxide laser system| Primary Device ID | 07290109146027 |
| NIH Device Record Key | 2d7dfe80-ed07-451c-bf01-f8a3f3f30a6a |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | AcuPulse™ DUO |
| Version Model Number | AcuPulse™ DUO |
| Catalog Number | RG-1000000 |
| Company DUNS | 600166524 |
| Company Name | LUMENIS LTD. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 07290109146027 [Primary] |
FDA Product Code
| GEX | Powered Laser Surgical Instrument |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-08-02 |
| Device Publish Date | 2022-07-25 |
On-Brand Devices [AcuPulse™ DUO]
| 07290109140292 | AcuPulse™ DUO |
| 07290109146027 | AcuPulse™ DUO |
| 07290109147239 | AcuPulse™ DUO |
Trademark Results [AcuPulse]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ACUPULSE 85331090 4080128 Dead/Cancelled |
ACUPOLL RESEARCH, INC. 2011-05-26 |
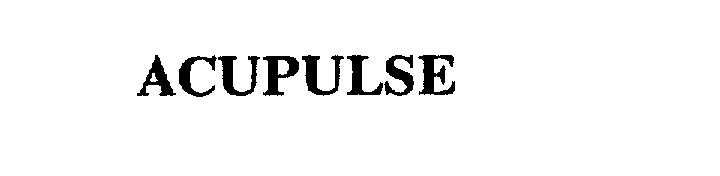 ACUPULSE 75663817 2332207 Dead/Cancelled |
AcuPOLL Precision Research 1999-03-19 |
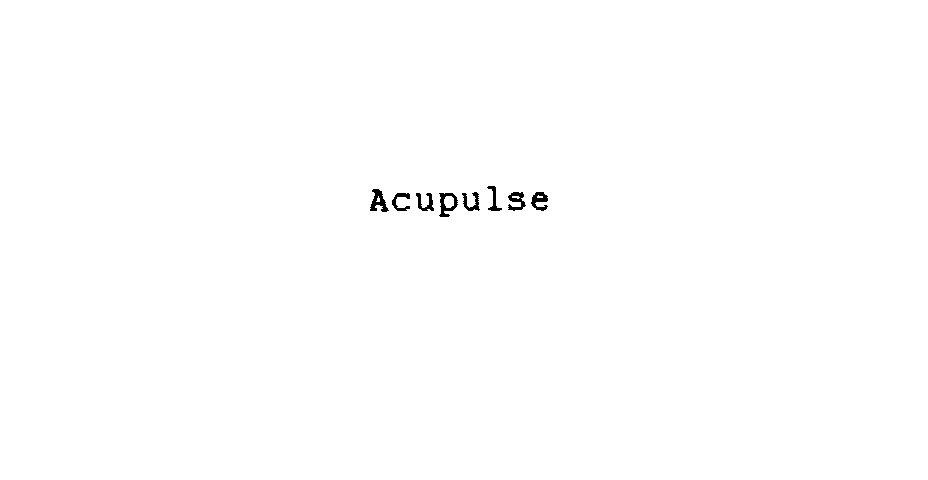 ACUPULSE 74541836 not registered Dead/Abandoned |
Acunetics Corporation 1994-06-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.