HemaPrep
GUDID 07350040972304
Blood smear instrument
Cellavision AB
Blood smear instrument IVD, manual| Primary Device ID | 07350040972304 |
| NIH Device Record Key | c3a907f4-56e8-465d-afe8-0f5209da36d9 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | HemaPrep |
| Version Model Number | XU-10012-01 |
| Company DUNS | 508663101 |
| Company Name | Cellavision AB |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +46464601600 |
| service@cellavision.se | |
| Phone | +46464601600 |
| service@cellavision.se | |
| Phone | +46464601600 |
| service@cellavision.se | |
| Phone | +46464601600 |
| service@cellavision.se | |
| Phone | +46464601600 |
| service@cellavision.se | |
| Phone | +46464601600 |
| service@cellavision.se | |
| Phone | +46464601600 |
| service@cellavision.se | |
| Phone | +46464601600 |
| service@cellavision.se | |
| Phone | +46464601600 |
| service@cellavision.se | |
| Phone | +46464601600 |
| service@cellavision.se |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 07350040972304 [Primary] |
FDA Product Code
| GKJ | Spinner, Slide, Automated |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2019-04-12 |
| Device Publish Date | 2018-11-07 |
Devices Manufactured by Cellavision AB
| 07350040975091 - CellaVision DC-1 | 2020-10-30 |
| 07350040975107 - CellaVision DC-1 PPA | 2020-10-30 |
| 07350040970201 - CellaVision® DM96 | 2019-04-12 Automated cell locating device. DM96 automatically locates and presents images of blood cells on peripherall blood smears. |
| 07350040971277 - CellaVision® DM1200 | 2019-04-12 Automated cell locating device. DM1200 automatically locates and presents images of blood cells on peripherall blood smears. |
| 07350040972021 - Automated Digital Morphology Analyzer DI-60 | 2019-04-12 Automated Digital Morphology Analyzer DI-60 automaticall locates and presents images of blood cells on peripheral blood smears |
| 07350040972304 - HemaPrep | 2019-04-12Blood smear instrument |
| 07350040972304 - HemaPrep | 2019-04-12 Blood smear instrument |
| 07350040972557 - CellaVision® Body Fluid Application DM1200 | 2019-04-12 The body fluid application automatically locates and presents cell images on cytocentrifuged body fluid preparations. |
| 07350040972564 - CellaVision® Body Fluid Application DM96 | 2019-04-12 The body fluid application automatically locates and presents cell images on cytocentrifuged body fluid preparations. |
Trademark Results [HemaPrep]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
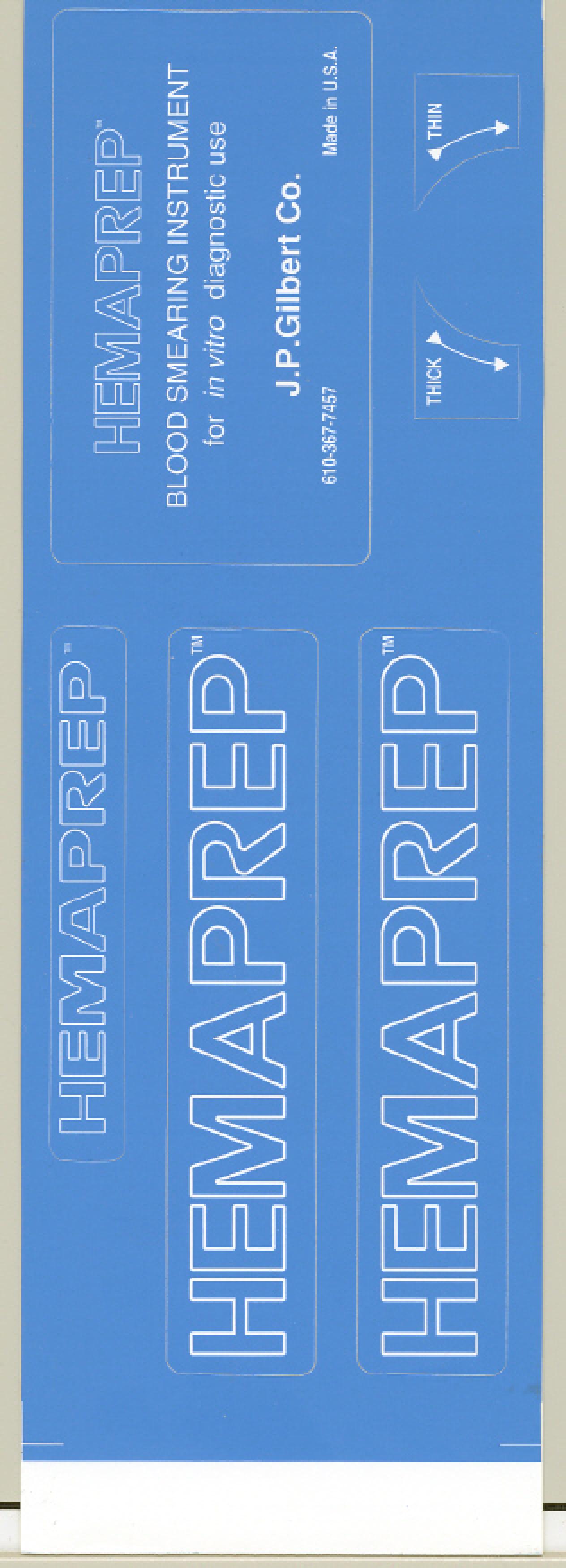 HEMAPREP 75280260 2219639 Live/Registered |
J.P. GILBERT COMPANY, INC. 1997-04-24 |
 HEMAPREP 73015463 1005771 Dead/Expired |
GEOMETRIC DATA CORPORATION 1974-03-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.