SPIRO 705-10
GUDID 07350061231077
SPIRO Tweezers
Fogless International AB
Dressing/utility forceps, tweezers-like, reusable| Primary Device ID | 07350061231077 |
| NIH Device Record Key | 0567393e-e531-4355-989b-97444febd7c9 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | SPIRO |
| Version Model Number | 705 |
| Catalog Number | 705-10 |
| Company DUNS | 356432815 |
| Company Name | Fogless International AB |
| Device Count | 2 |
| DM Exempt | true |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +4636144100 |
| mail@fogless.se | |
| Phone | +4636144100 |
| mail@fogless.se | |
| Phone | +4636144100 |
| mail@fogless.se | |
| Phone | +4636144100 |
| mail@fogless.se | |
| Phone | +4636144100 |
| mail@fogless.se | |
| Phone | +4636144100 |
| mail@fogless.se | |
| Phone | +4636144100 |
| mail@fogless.se | |
| Phone | +4636144100 |
| mail@fogless.se | |
| Phone | +4636144100 |
| mail@fogless.se | |
| Phone | +4636144100 |
| mail@fogless.se | |
| Phone | +4636144100 |
| mail@fogless.se | |
| Phone | +4636144100 |
| mail@fogless.se | |
| Phone | +4636144100 |
| mail@fogless.se | |
| Phone | +4636144100 |
| mail@fogless.se | |
| Phone | +4636144100 |
| mail@fogless.se |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dark and dry environment at normal indoor room temperature |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dark and dry environment at normal indoor room temperature |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dark and dry environment at normal indoor room temperature |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dark and dry environment at normal indoor room temperature |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dark and dry environment at normal indoor room temperature |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dark and dry environment at normal indoor room temperature |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dark and dry environment at normal indoor room temperature |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dark and dry environment at normal indoor room temperature |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dark and dry environment at normal indoor room temperature |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dark and dry environment at normal indoor room temperature |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dark and dry environment at normal indoor room temperature |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dark and dry environment at normal indoor room temperature |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dark and dry environment at normal indoor room temperature |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dark and dry environment at normal indoor room temperature |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dark and dry environment at normal indoor room temperature |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 07350061231060 [Primary] |
| GS1 | 07350061231077 [Package] Contains: 07350061231060 Package: Zip-Lock Bag [5 Units] In Commercial Distribution |
| GS1 | 07350061231312 [Unit of Use] |
FDA Product Code
| GEN | Forceps, General & Plastic Surgery |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2018-06-11 |
| Device Publish Date | 2018-03-31 |
On-Brand Devices [SPIRO]
| 07350061231350 | SPIRO Filter |
| 07350061231282 | SPIRO Basic Set Humidifier |
| 07350061231268 | SPIRO Basic Set Speaking Valve |
| 07350061231237 | Shower Protection for SPIRO Speaking Valve models 710,710R,711 |
| 07350061231183 | SPIRO Speaking Valve Without Oxygen Nipple |
| 07350061231152 | SPIRO Speaking Valve With Oxygen Nipple (Red) |
| 07350061231121 | SPIRO Speaking Valve With Oxygen Nipple |
| 07350061231114 | SPIRO Adapter (white) 14mm for SPIRO 702, 710, 710R and 711 to connect to a non ISO 15 tapered c |
| 07350061231091 | SPIRO Adapter (transparent) 13mm for SPIRO 702, 710, 710R and 711 to connect to a non ISO 15 tap |
| 07350061231077 | SPIRO Tweezers |
| 07350061231046 | Shower Protector for SPIRO 702 Humidifier |
| 07350061231008 | SPIRO 702 Humidifier |
| 07350061230353 | XXX |
Trademark Results [SPIRO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SPIRO 97777521 not registered Live/Pending |
Flannery, Danielle 2023-02-02 |
 SPIRO 97250079 not registered Live/Pending |
Noxtak Corp. 2022-02-02 |
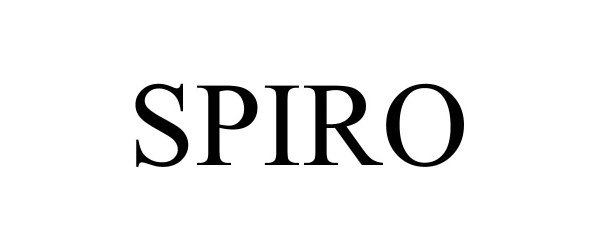 SPIRO 97230507 not registered Live/Pending |
GLOBAL EXPERIENCE SPECIALISTS, INC. 2022-01-20 |
 SPIRO 97230432 not registered Live/Pending |
GLOBAL EXPERIENCE SPECIALISTS, INC. 2022-01-20 |
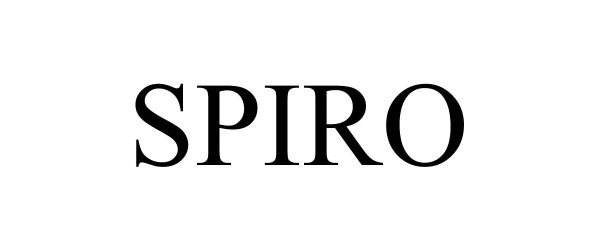 SPIRO 97230396 not registered Live/Pending |
GLOBAL EXPERIENCE SPECIALISTS, INC. 2022-01-20 |
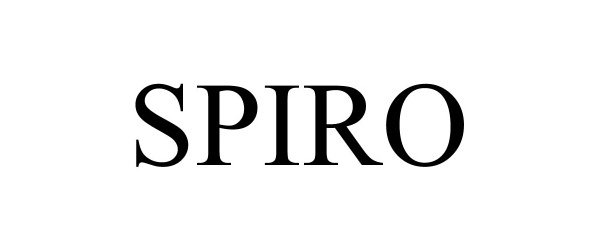 SPIRO 97230226 not registered Live/Pending |
GLOBAL EXPERIENCE SPECIALISTS, INC. 2022-01-20 |
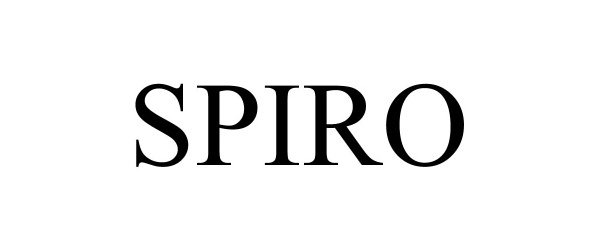 SPIRO 97230177 not registered Live/Pending |
GLOBAL EXPERIENCE SPECIALISTS, INC. 2022-01-20 |
 SPIRO 97230090 not registered Live/Pending |
GLOBAL EXPERIENCE SPECIALISTS, INC. 2022-01-20 |
 SPIRO 97229902 not registered Live/Pending |
GLOBAL EXPERIENCE SPECIALISTS, INC. 2022-01-20 |
 SPIRO 97117258 not registered Live/Pending |
Spiro LLC 2021-11-10 |
 SPIRO 90399208 not registered Live/Pending |
STRECHR, LLC 2020-12-21 |
 SPIRO 88850622 not registered Live/Pending |
Nicopure Labs, LLC 2020-03-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.