M-SG® Star 1 05000433
GUDID 07640166515098
M-SG® Star 1 Female part T
Cendres+Métaux SA
Sliding dental precision attachment| Primary Device ID | 07640166515098 |
| NIH Device Record Key | 7249e362-fce0-4f9a-ad7e-5bf1ff9b4b9c |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | M-SG® Star 1 |
| Version Model Number | 05000433 |
| Catalog Number | 05000433 |
| Company DUNS | 487506230 |
| Company Name | Cendres+Métaux SA |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch | |
| Phone | +41583602000 |
| info@cmsa.ch |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep away from sunlight |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 07640166515098 [Primary] |
FDA Product Code
| EGG | Attachment, Precision, All |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
[07640166515098]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-09-14 |
| Device Publish Date | 2023-09-06 |
On-Brand Devices [M-SG® Star 1]
| 07640166515197 | M-SG® Star 1 Insert remover |
| 07640166515173 | M-SG® Star 1 Duplicating aid G |
| 07640166515098 | M-SG® Star 1 Female part T |
| 07640166515067 | M-SG® Star 1 TC for casting-on |
Trademark Results [M-SG]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
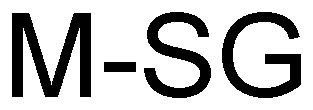 M-SG 79032210 3382132 Dead/Cancelled |
Cendres+Métaux Holding SA 2006-11-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.