GALILEI 410.030.007
GUDID 07640167751242
Galilei G6 Lens Professional
SIS AG, Surgical Instrument Systems
Optical low coherence reflectometry (OLCR) biometer| Primary Device ID | 07640167751242 |
| NIH Device Record Key | f52a5a82-27db-4816-9e81-3a892ba413ea |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | GALILEI |
| Version Model Number | G6 |
| Catalog Number | 410.030.007 |
| Company DUNS | 480508261 |
| Company Name | SIS AG, Surgical Instrument Systems |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 8667084490 |
| usa@ziemergroup.com |
Operating and Storage Conditions
| Handling Environment Atmospheric Pressure | Between 690 millibar and 1060 millibar |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 07640167751242 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K182659
FDA Product Code
| MXK | Device, Analysis, Anterior Segment |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2019-09-30 |
| Device Publish Date | 2019-09-20 |
On-Brand Devices [GALILEI]
| 07640167751235 | Galilei Dual Scheimpflug Analyzer |
| 07640167751242 | Galilei G6 Lens Professional |
Trademark Results [GALILEI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
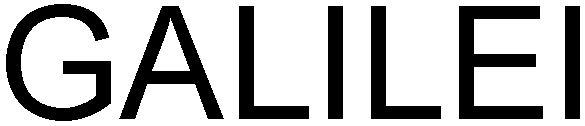 GALILEI 79109198 4319357 Live/Registered |
Ziemer Ophthalmic Systems AG 2011-12-12 |
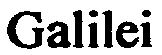 GALILEI 79029614 not registered Dead/Abandoned |
Peter Herres; Wein- und Sektkellerei GmbH, Trier 2006-08-28 |
 GALILEI 78698103 not registered Dead/Abandoned |
SIS AG 2005-08-23 |
 GALILEI 78676027 not registered Dead/Abandoned |
SIS Ltd 2005-07-22 |
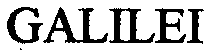 GALILEI 76447848 not registered Dead/Abandoned |
RAGATZ, ANTONIO 2002-09-06 |
 GALILEI 74260195 not registered Dead/Abandoned |
Muelhens KG 1992-03-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.