Starter
GUDID 07640181161522
Keri Medical SA
Orthopaedic surgical procedure kit, non-medicated, reusable| Primary Device ID | 07640181161522 |
| NIH Device Record Key | f2232e42-2418-4a54-bcff-cf914a525182 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Starter |
| Version Model Number | 211-A30000 |
| Company DUNS | 480138827 |
| Company Name | Keri Medical SA |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 07640181161522 [Primary] |
FDA Product Code
| HWD | Starter, Bone Screw |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[07640181161522]
Moist Heat or Steam Sterilization
[07640181161522]
Moist Heat or Steam Sterilization
[07640181161522]
Moist Heat or Steam Sterilization
[07640181161522]
Moist Heat or Steam Sterilization
[07640181161522]
Moist Heat or Steam Sterilization
[07640181161522]
Moist Heat or Steam Sterilization
[07640181161522]
Moist Heat or Steam Sterilization
[07640181161522]
Moist Heat or Steam Sterilization
[07640181161522]
Moist Heat or Steam Sterilization
[07640181161522]
Moist Heat or Steam Sterilization
[07640181161522]
Moist Heat or Steam Sterilization
[07640181161522]
Moist Heat or Steam Sterilization
[07640181161522]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-11-30 |
| Device Publish Date | 2022-11-22 |
Devices Manufactured by Keri Medical SA
Trademark Results [Starter]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 STARTER 98395353 not registered Live/Pending |
Brawndo LLC 2024-02-07 |
 STARTER 97839301 not registered Live/Pending |
Icon DE Holdings LLC 2023-03-14 |
 STARTER 97237051 not registered Live/Pending |
Icon DE Holdings LLC 2022-01-25 |
 STARTER 90787494 not registered Live/Pending |
Starter App LLC 2021-06-22 |
 STARTER 90574745 not registered Live/Pending |
Xiamen Uni-Green Industries Co.,Ltd 2021-03-11 |
 STARTER 88588837 not registered Live/Pending |
Icon DE Holdings LLC 2019-08-22 |
 STARTER 88174313 not registered Live/Pending |
ICON DE HOLDINGS LLC 2018-10-30 |
 STARTER 87635405 5758483 Live/Registered |
ICON DE HOLDINGS LLC 2017-10-05 |
 STARTER 86910723 not registered Live/Pending |
Icon DE Holdings LLC 2016-02-17 |
 STARTER 86158400 4946687 Live/Registered |
Kai U.S.A., Ltd. 2014-01-06 |
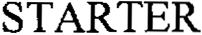 STARTER 79108837 not registered Dead/Abandoned |
Z Marine International S.Ã .r.l. 2011-12-08 |
 STARTER 78889782 not registered Dead/Abandoned |
PlayStar, Inc. 2006-05-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.