SYNECOM
GUDID 08031527013435
MICROPORT CRM SRL
Electrocardiographic long-term ambulatory recording analyser| Primary Device ID | 08031527013435 |
| NIH Device Record Key | 215e1f20-4128-4ada-8e13-e8c2b98e4937 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | SYNECOM |
| Version Model Number | V3.10 |
| Company DUNS | 438860859 |
| Company Name | MICROPORT CRM SRL |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 08031527013435 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K042002
FDA Product Code
| DQK | COMPUTER, DIAGNOSTIC, PROGRAMMABLE |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2020-10-20 |
| Device Publish Date | 2016-09-22 |
Devices Manufactured by MICROPORT CRM SRL
| 08031527019611 - CPR4 | 2023-07-04 Inductive Telemetry Head CPR4 USB |
| 08031527019819 - Vega | 2023-07-04 VEGA Stylet Kit 45cm |
| 08031527019826 - Vega | 2023-07-04 VEGA Stylet Kit 52cm |
| 08031527019833 - Vega | 2023-07-04 VEGA Stylet Kit 58cm |
| 08031527018997 - BlueSky | 2023-07-03 BlueSky DR Alizea 1600 |
| 08031527019000 - BlueSky | 2023-07-03 BlueSky SR Alizea 1300 |
| 08031527019017 - BlueSky | 2023-07-03 BlueSky DR Celea 1400 |
| 08031527019024 - BlueSky | 2023-07-03 BlueSky SR Celea 1100 |
Trademark Results [SYNECOM]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
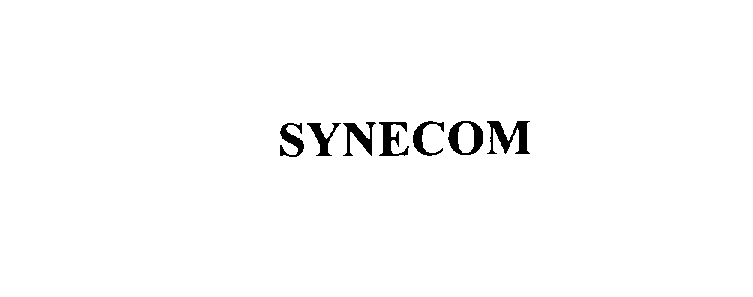 SYNECOM 76115695 2608804 Dead/Cancelled |
Ela Medical 2000-08-23 |
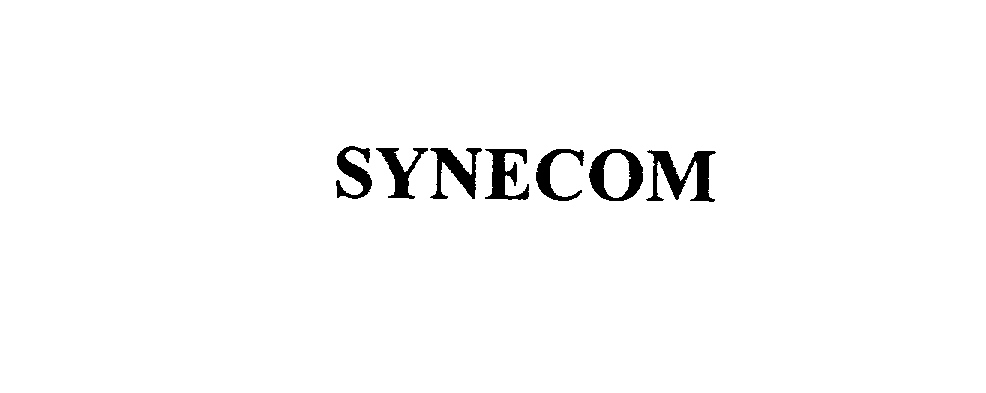 SYNECOM 76085642 not registered Dead/Abandoned |
Synecom Communications Corporation 2000-07-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.