Symbioso
GUDID 08592654104880
active integrated anti-decubitus mattresses
L I N E T spol. s r.o.
Alternating-pressure bed mattress overlay system| Primary Device ID | 08592654104880 |
| NIH Device Record Key | 0cf91af8-eb71-4c1a-a451-51d784fa074d |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Symbioso |
| Version Model Number | 1VSR000265000 |
| Company DUNS | 644544504 |
| Company Name | L I N E T spol. s r.o. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 08592654104880 [Primary] |
FDA Product Code
| FNM | Mattress, Air Flotation, Alternating Pressure |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-07-06 |
| Device Publish Date | 2023-06-28 |
On-Brand Devices [Symbioso]
| 08592654104880 | active integrated anti-decubitus mattresses |
| 08592654403211 | active integrated anti-decubitus mattresses |
Trademark Results [Symbioso]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
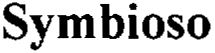 SYMBIOSO 79135319 4642924 Live/Registered |
LINET, spol. s r.o. 2013-05-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.