Passport
GUDID 08714729154921
Balloon Dilatation Catheter
BOSTON SCIENTIFIC CORPORATION
Urethral dilatation catheter, non-medicated| Primary Device ID | 08714729154921 |
| NIH Device Record Key | fa948208-5354-4ef8-bf8c-eb6b1b67ec85 |
| Commercial Distribution Discontinuation | 2023-05-15 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | Passport |
| Version Model Number | M0062181000 |
| Company DUNS | 021717889 |
| Company Name | BOSTON SCIENTIFIC CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 08714729154921 [Primary] |
FDA Product Code
| EZN | DILATOR, CATHETER, URETERAL |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2024-04-05 |
| Device Publish Date | 2016-09-24 |
On-Brand Devices [Passport]
| 08714729191872 | Balloon Dilatation Catheter |
| 08714729154969 | Balloon Dilatation Catheter |
| 08714729154921 | Balloon Dilatation Catheter |
| 08714729042549 | Balloon Dilatation Catheter |
Trademark Results [Passport]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PASSPORT 98809085 not registered Live/Pending |
Unblocked Brands, Inc. 2024-10-18 |
 PASSPORT 98747634 not registered Live/Pending |
GroPro Corporation 2024-09-12 |
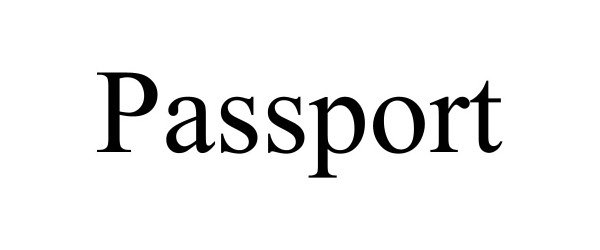 PASSPORT 98669203 not registered Live/Pending |
Beyond Earth, LLC 2024-07-26 |
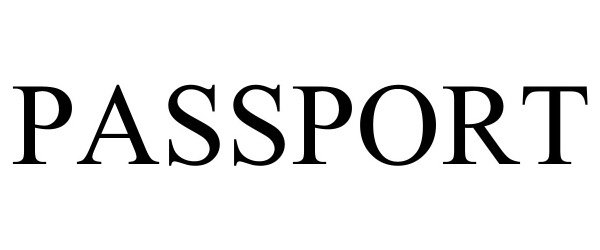 PASSPORT 98176230 not registered Live/Pending |
Amazon Technologies, Inc. 2023-09-12 |
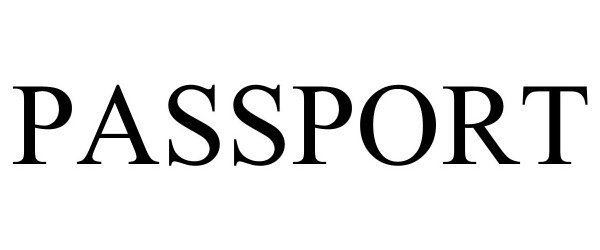 PASSPORT 98087314 not registered Live/Pending |
Passport, Inc. 2023-07-17 |
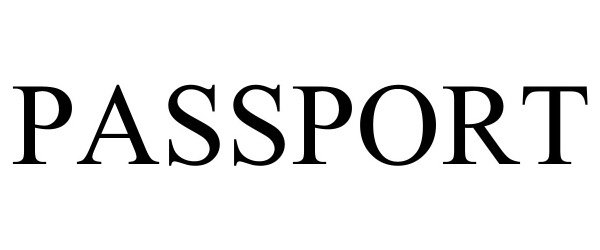 PASSPORT 98045988 not registered Live/Pending |
777ABC, LLC 2023-06-16 |
 PASSPORT 98028348 not registered Live/Pending |
Circonus, Inc. 2023-06-05 |
 PASSPORT 98028171 not registered Live/Pending |
CNA Financial Corporation 2023-06-05 |
 PASSPORT 97792011 not registered Live/Pending |
Crewfare LLC 2023-02-13 |
 PASSPORT 97607747 not registered Live/Pending |
MillerKnoll, Inc. 2022-09-26 |
 PASSPORT 97551854 not registered Live/Pending |
Pelco Products, Inc. 2022-08-17 |
 PASSPORT 97470517 not registered Live/Pending |
ProviderTrust, Inc. 2022-06-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.