Transpara
GUDID 08719326450124
The ScreenPoint Transpara system is intended for use as a concurrent reading aid for physicians interpreting screening mammograms from compatible FFDM systems, to identify regions suspicious for breast cancer and assess their likelihood of malignancy. Output of the device includes marks placed on suspicious soft tissue lesions and suspicious calcifications, region based scores, displayed upon the physicians query, indicating the likelihood that cancer is present in specific regions, and an overall score indicating the likelihood that cancer is present on the mammogram. Patient management decisions should not be made solely on the basis of analysis by Transpara.
Screenpoint Medical B.V.
X-ray image interpretive software| Primary Device ID | 08719326450124 |
| NIH Device Record Key | ccabcc03-a9cd-4514-ad9c-c9cb0aedd382 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Transpara |
| Version Model Number | 1.5.0 2D |
| Company DUNS | 491352982 |
| Company Name | Screenpoint Medical B.V. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 08719326450124 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K192287
FDA Product Code
| QDQ | Radiological Computer Assisted Detection/Diagnosis Software For Lesions Suspicious For Cancer |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2019-12-20 |
| Device Publish Date | 2019-12-12 |
On-Brand Devices [Transpara]
| 08719326450100 | The ScreenPoint Transpara system is intended for use as a concurrent reading aid for physicians |
| 08719326450124 | The ScreenPoint Transpara system is intended for use as a concurrent reading aid for physicians |
| 08719326450117 | Transpara™ software is intended for use as a concurrent reading aid for physicians interpretin |
| 08719326450131 | Transpara® software is intended for use as a concurrent reading aid for physicians interpreting |
| 08720387058013 | Transpara® software is intended for use as a concurrent reading aid for physicians interpreting |
| 08720387058044 | Transpara® software is intended for use as a concurrent reading aid for physicians interpreting |
| 08720387058051 | Transpara® software is intended for use as a concurrent reading aid for physicians interpreting |
Trademark Results [Transpara]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
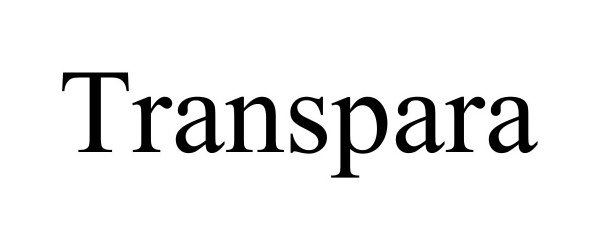 TRANSPARA 97560177 not registered Live/Pending |
CPS Technology Holdings LLC 2022-08-23 |
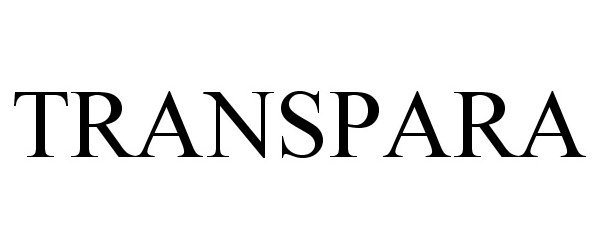 TRANSPARA 87822676 5906201 Live/Registered |
Transpara, LLC 2018-03-06 |
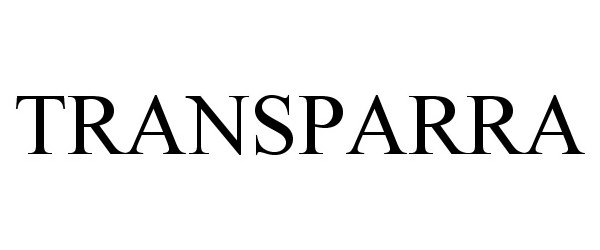 TRANSPARA 87012066 not registered Dead/Abandoned |
C.R. Laurence Co., Inc. 2016-04-24 |
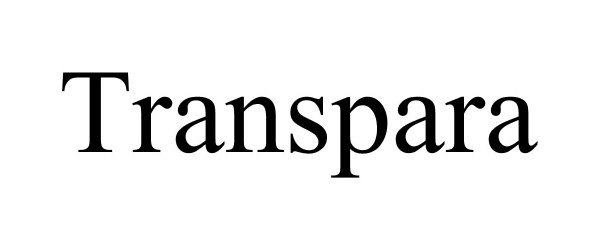 TRANSPARA 86756857 4968025 Live/Registered |
ScreenPoint Medical BV 2015-09-15 |
 TRANSPARA 77818070 not registered Dead/Abandoned |
Matakina Technology Limited 2009-09-02 |
 TRANSPARA 71592472 0541135 Dead/Expired |
BURLINGTON MILLS CORPORATION 1950-02-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.