LIPOcel
GUDID 08800020100009
Focused Ultrasound for tissue heat or mechanical cellular disruption
Jeisys Medical Inc.
Ultrasound hyperthermia system transducer, extracorporeal| Primary Device ID | 08800020100009 |
| NIH Device Record Key | fe2108be-46b0-4f60-9c9a-db5c9fa9b8cf |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | LIPOcel |
| Version Model Number | LIPOcel |
| Company DUNS | 690275362 |
| Company Name | Jeisys Medical Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 08800020100009 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K181896
FDA Product Code
| OHV | Focused Ultrasound For Tissue Heat Or Mechanical Cellular Disruption |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-03-04 |
| Device Publish Date | 2020-02-25 |
Devices Manufactured by Jeisys Medical Inc.
| 08800020102836 - JRF-Pad-01 | 2025-12-18 JRF-Pad-01 |
| 08800020106759 - Jeisys Medical Inc. | 2025-09-05 DENSITY Noir Main body |
| 08800020106407 - Jeisys Medical Inc. | 2025-09-02 ALPHA Tip |
| 18800020105506 - POTENZA Tip | 2025-05-27 POTENZA Tip (Sterile micro needle electrode CP-25 Tip) |
| 18800020105612 - POTENZA Tip | 2025-05-27 POTENZA Tip (Sterile micro needle electrode CP-16 Tip) |
| 18800020105629 - POTENZA Tip | 2025-05-27 POTENZA Tip (Sterile micro needle electrode CP-49 Tip) |
| 18800020106299 - POTENZA Tip | 2025-05-27 POTENZA Tip (DDR Tip) |
| 08800020106308 - POTENZA Tip | 2025-05-27 POTENZA Tip (SFA Tip) |
Trademark Results [LIPOcel]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LIPOCEL 98123239 not registered Live/Pending |
JEISYS MEDICAL INC. 2023-08-08 |
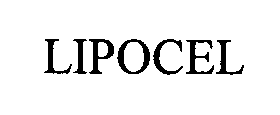 LIPOCEL 76475906 2971673 Dead/Cancelled |
MAXIMUM HUMAN PERFORMANCE, LLC 2002-12-18 |
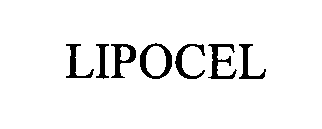 LIPOCEL 76475905 2826191 Dead/Cancelled |
MAXIMUM HUMAN PERFORMANCE, LLC 2002-12-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.