HDX2
GUDID 08806392040097
INTEROJO INC.
Soft corrective contact lens, daily-wear| Primary Device ID | 08806392040097 |
| NIH Device Record Key | 60802446-5ddc-4777-91f3-64dbd970e010 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | HDX2 |
| Version Model Number | HDX2 |
| Company DUNS | 688455799 |
| Company Name | INTEROJO INC. |
| Device Count | 6 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1-760-754-2220 |
| rho@interojo.com |
Operating and Storage Conditions
| Storage Environment Temperature | Between 1 Degrees Celsius and 30 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 08806392040097 [Primary] |
| GS1 | 08806392040219 [Unit of Use] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K080794
FDA Product Code
| LPL | Lenses, Soft Contact, Daily Wear |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-02-11 |
| Device Publish Date | 2020-02-03 |
Devices Manufactured by INTEROJO INC.
| 08809752409763 - HAPA KRISTIN | 2025-04-28 |
| 08809752409770 - HAPA KRISTIN | 2025-04-28 |
| 08809752409787 - HAPA KRISTIN | 2025-04-28 |
| 08809752409794 - HAPA KRISTIN | 2025-04-28 |
| 08800245547405 - Miz | 2024-10-02 |
| 08800245547412 - Miz | 2024-10-02 |
| 08800245547429 - Miz | 2024-10-02 |
| 08800245547436 - Miz | 2024-10-02 |
Trademark Results [HDX2]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
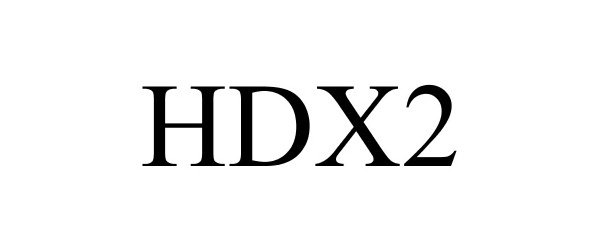 HDX2 87251705 not registered Dead/Abandoned |
Trivinci Systems, LLC 2016-11-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.