E-CUBE
GUDID 08809260470910
20:1, EP Type E-CUBE(C/B)+CUBE-EP+ACL(B)-42EP
Saeshin Precision Co., Ltd.
Dental power tool system, line-powered| Primary Device ID | 08809260470910 |
| NIH Device Record Key | 9771e97f-59de-40d4-b4c7-6f91d5fa21c1 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | E-CUBE |
| Version Model Number | E-CUBE #1 |
| Company DUNS | 688019566 |
| Company Name | Saeshin Precision Co., Ltd. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 08809260470910 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K111616
FDA Product Code
| EKX | Handpiece, Direct Drive, Ac-Powered |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[08809260470910]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-01-30 |
| Device Publish Date | 2020-01-22 |
On-Brand Devices [E-CUBE]
| 08809260470910 | 20:1, EP Type E-CUBE(C/B)+CUBE-EP+ACL(B)-42EP |
| 08809260470903 | 20:1, EM Type E-CUBE(C/B)+CUBE-EM+ACL(B)-42EM |
| 08809260470514 | Angle Handpiece |
| 08809260470507 | Angle Handpiece |
| 08809260470491 | Implant Motor |
| 08809260470484 | Implant Motor |
| 08809260470477 | Control Box |
Trademark Results [E-CUBE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
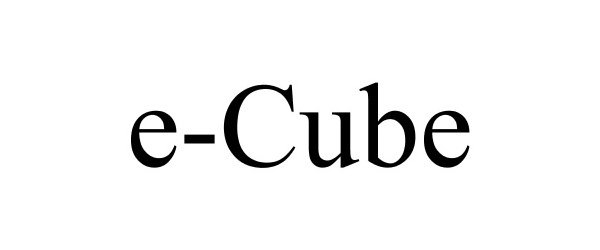 E-CUBE 86224124 4846854 Live/Registered |
B+ Equipment 2014-03-18 |
 E-CUBE 79143962 4910633 Live/Registered |
Eisenmann SE 2013-11-06 |
 E-CUBE 79092640 4058236 Live/Registered |
ALPINION MEDICAL SYSTEMS CO., LTD. 2010-10-08 |
 E-CUBE 78153455 not registered Dead/Abandoned |
Encoder Products Company, Inc. 2002-08-12 |
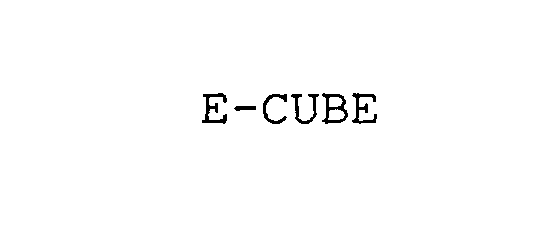 E-CUBE 76112060 not registered Dead/Abandoned |
Nagase & Co., Ltd. 2000-08-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.