Mygen™ RF Generator M-3004
GUDID 08809295147023
RF Medical Co.,Ltd.
Percutaneous radio-frequency ablation system generator| Primary Device ID | 08809295147023 |
| NIH Device Record Key | bc3d6894-3976-41f3-b49e-492578ac03e6 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Mygen™ RF Generator M-3004 |
| Version Model Number | M-3004 |
| Company DUNS | 690514869 |
| Company Name | RF Medical Co.,Ltd. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 08809295147023 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K221277
FDA Product Code
| GEI | Electrosurgical, Cutting & Coagulation & Accessories |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2025-09-15 |
| Device Publish Date | 2025-09-05 |
Devices Manufactured by RF Medical Co.,Ltd.
| 08800142906435 - BIG-TIP™ | 2025-09-15 |
| 08800142906442 - BIG-TIP™ | 2025-09-15 |
| 08800142906459 - BIG-TIP™ | 2025-09-15 |
| 08800142906534 - BIG-TIP™ | 2025-09-15 |
| 08800142906541 - BIG-TIP™ | 2025-09-15 |
| 08800142906558 - BIG-TIP™ | 2025-09-15 |
| 08800142906565 - BIG-TIP™ | 2025-09-15 |
| 08800142906572 - BIG-TIP™ | 2025-09-15 |
Trademark Results [Mygen]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
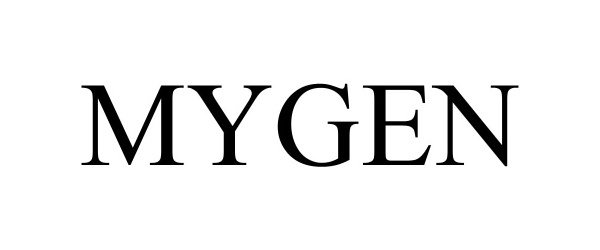 MYGEN 98275785 not registered Live/Pending |
RF Medical Co., Ltd. 2023-11-17 |
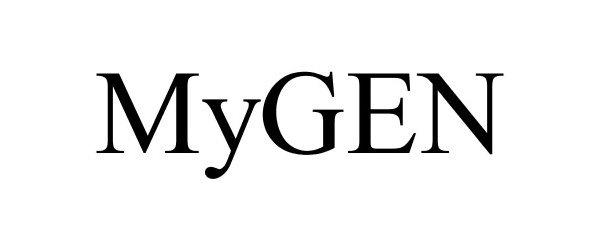 MYGEN 88339586 not registered Live/Pending |
Nucorplabs, Inc. 2019-03-14 |
 MYGEN 79221198 5658427 Live/Registered |
Clear Blue Energy Limited 2016-12-22 |
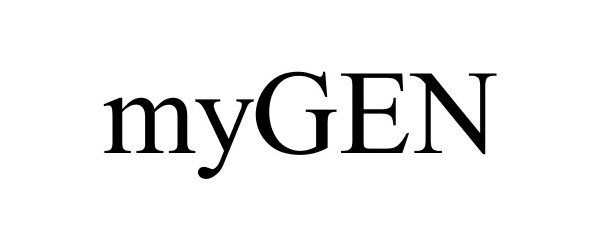 MYGEN 77891436 3862044 Dead/Cancelled |
Maxell Corporation of America 2009-12-11 |
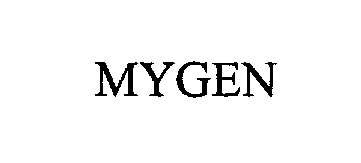 MYGEN 76237782 2617422 Live/Registered |
Kyocera Solar, Inc. 2001-04-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.