WF-100
GUDID 08809520341509
GTG Wellness Co., Ltd.
Hand-held electric massager| Primary Device ID | 08809520341509 |
| NIH Device Record Key | 49b32406-d42a-438c-b17e-3250444f73d0 |
| Commercial Distribution Discontinuation | 2023-03-17 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | WF-100 |
| Version Model Number | WF-100 Fullset |
| Company DUNS | 689458057 |
| Company Name | GTG Wellness Co., Ltd. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 08809520341509 [Primary] |
FDA Product Code
| ISA | Massager, Therapeutic, Electric |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-03-27 |
| Device Publish Date | 2023-03-17 |
Devices Manufactured by GTG Wellness Co., Ltd.
| 08809520340014 - LEBODY FORM | 2023-03-27 |
| 08809520341004 - OLG-200 | 2023-03-27 |
| 08809520341011 - OLG-200 | 2023-03-27 |
| 08809520341028 - OLG-200 | 2023-03-27 |
| 08809520341035 - OLG-100 | 2023-03-27 |
| 08809520341042 - OLG-100 | 2023-03-27 |
| 08809520341066 - OLG-100 | 2023-03-27 |
| 08809520341103 - OLZ-200 | 2023-03-27 |
Trademark Results [WF-100]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
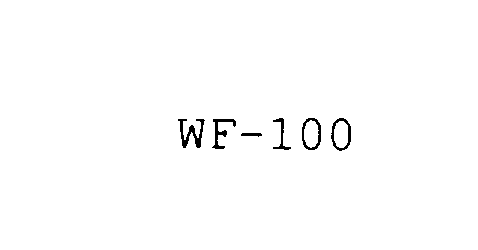 WF-100 78001822 2541772 Dead/Cancelled |
Mallinckrodt Inc. 2000-03-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.