Slim Line EBS202010
GUDID 08809691368251
Endosseous dental implant
Dentium Co., Ltd.
Screw endosteal dental implant, one-piece| Primary Device ID | 08809691368251 |
| NIH Device Record Key | 23db8928-9777-4407-8549-c4d2440e2566 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Slim Line |
| Version Model Number | EBS202010 |
| Catalog Number | EBS202010 |
| Company DUNS | 689514395 |
| Company Name | Dentium Co., Ltd. |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | MR Conditional |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(714)226-0229 |
| cs@dentiumusa.com | |
| Phone | +1(714)226-0229 |
| cs@dentiumusa.com | |
| Phone | +1(714)226-0229 |
| cs@dentiumusa.com | |
| Phone | +1(714)226-0229 |
| cs@dentiumusa.com | |
| Phone | +1(714)226-0229 |
| cs@dentiumusa.com | |
| Phone | +1(714)226-0229 |
| cs@dentiumusa.com | |
| Phone | +1(714)226-0229 |
| cs@dentiumusa.com | |
| Phone | +1(714)226-0229 |
| cs@dentiumusa.com | |
| Phone | +1(714)226-0229 |
| cs@dentiumusa.com |
Device Dimensions
| Device Size Text, specify | 0 |
| Length | 10 Millimeter |
| Device Size Text, specify | 0 |
| Length | 10 Millimeter |
| Device Size Text, specify | 0 |
| Length | 10 Millimeter |
| Device Size Text, specify | 0 |
| Length | 10 Millimeter |
| Device Size Text, specify | 0 |
| Length | 10 Millimeter |
| Device Size Text, specify | 0 |
| Length | 10 Millimeter |
| Device Size Text, specify | 0 |
| Length | 10 Millimeter |
| Device Size Text, specify | 0 |
| Length | 10 Millimeter |
| Device Size Text, specify | 0 |
| Length | 10 Millimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 08809691368251 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K111162
FDA Product Code
| DZE | IMPLANT, ENDOSSEOUS, ROOT-FORM |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-08-30 |
| Device Publish Date | 2021-08-20 |
On-Brand Devices [Slim Line]
| 08809162706735 | Accessories, Implant, Dental, Endosseousv |
| 08809162706728 | Accessories, Implant, Dental, Endosseous |
| 08809162706704 | Accessories, Implant, Dental, Endosseous |
| 08809162706681 | Accessories, Implant, Dental, Endosseous |
| 08809162706674 | Accessories, Implant, Dental, Endosseous |
| 08809162704830 | Accessories, Implant, Dental, Endosseous |
| 08809544905596 | Chisel, Osteotome, Surgical |
| 08809544905589 | Chisel, Osteotome, Surgical |
| 08809544905572 | Chisel, Osteotome, Surgical |
| 08809544905565 | Chisel, Osteotome, Surgical |
| 08809544902236 | Accessories, Implant, Dental, Endosseous |
| 08809460301748 | Chisel, Osteotome, Surgical |
| 08809162706759 | Chisel, Osteotome, Surgical |
| 08809544907491 | Accessories, Implant, Dental, Endosseous |
| 08809544907484 | Accessories, Implant, Dental, Endosseous |
| 08809691368619 | Endosseous dental implant abutment |
| 08809691368602 | Endosseous dental implant abutment |
| 08809691368374 | Endosseous dental implant |
| 08809691368367 | Endosseous dental implant |
| 08809691368350 | Endosseous dental implant |
| 08809691368329 | Endosseous dental implant |
| 08809691368312 | Endosseous dental implant |
| 08809691368305 | Endosseous dental implant |
| 08809691368275 | Endosseous dental implant |
| 08809691368268 | Endosseous dental implant |
| 08809691368251 | Endosseous dental implant |
| 08809691365786 | Endosseous dental implant |
| 08809691365779 | Endosseous dental implant |
| 08809691365748 | Endosseous dental implant |
| 08809691365731 | Endosseous dental implant |
| 08809691365724 | Endosseous dental implant |
| 08809691365694 | Endosseous dental implant |
| 08809691365687 | Endosseous dental implant |
| 08809691361122 | Endosseous dental implant |
| 08809691361115 | Endosseous dental implant |
Trademark Results [Slim Line]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
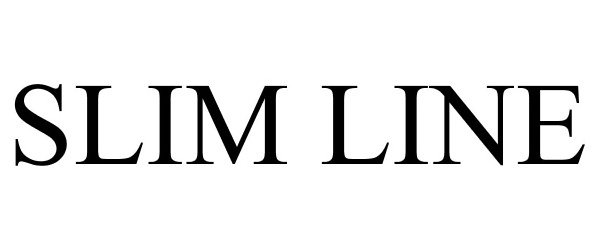 SLIM LINE 86593681 4858640 Live/Registered |
Flomatic Corporation 2015-04-10 |
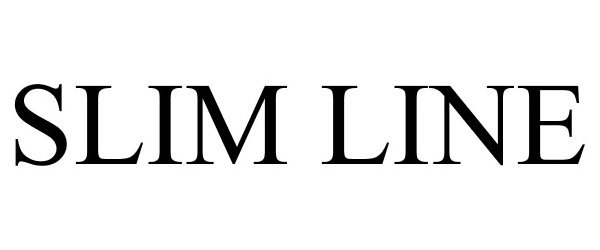 SLIM LINE 86588562 4858494 Live/Registered |
Chade Fashions, Inc. 2015-04-06 |
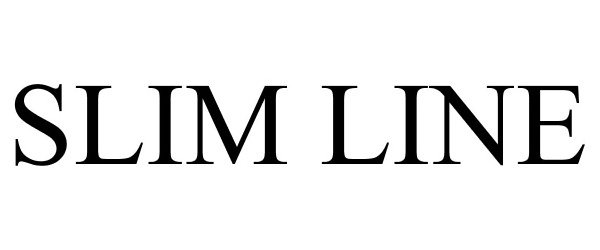 SLIM LINE 86143840 4698182 Live/Registered |
KK International Trading Corporation 2013-12-14 |
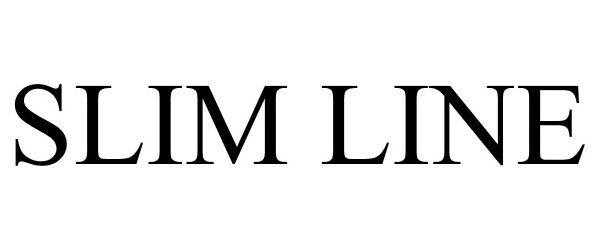 SLIM LINE 85442539 4153798 Live/Registered |
Live Eyewear, Inc. 2011-10-07 |
 SLIM LINE 85053134 3992278 Live/Registered |
DEDON GmbH 2010-06-02 |
 SLIM LINE 81035756 1035756 Dead/Cancelled |
Bulova Watch Company, Inc. 0000-00-00 |
 SLIM LINE 81029173 1029173 Dead/Cancelled |
Golconda Corporation 0000-00-00 |
 SLIM LINE 80995008 0995008 Dead/Cancelled |
Great State Mfg. Corp. 0000-00-00 |
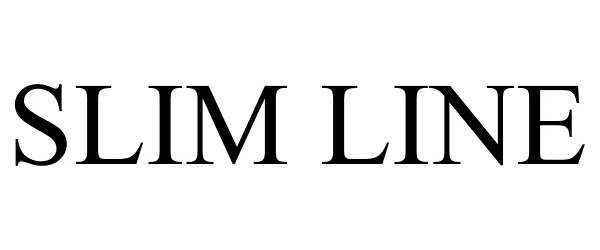 SLIM LINE 78635869 3126156 Dead/Cancelled |
Annwil Inc. 2005-05-24 |
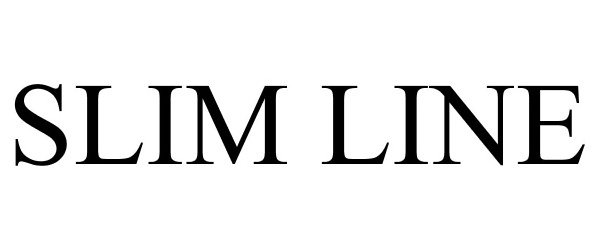 SLIM LINE 77068638 not registered Dead/Abandoned |
OFS FITEL, LLC 2006-12-20 |
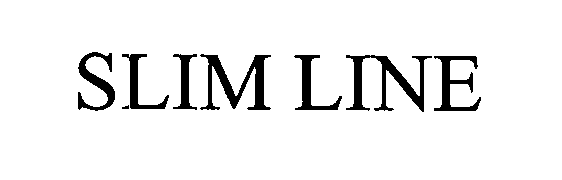 SLIM LINE 76538839 3056784 Dead/Cancelled |
COLE KEPRO INTERNATIONAL, LLC 2003-08-20 |
 SLIM LINE 76521853 not registered Dead/Abandoned |
FIBREFORM CONTAINERS, INC. 2003-06-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.