Sleeve 54101-80-2
GUDID 09120047306147
Self Holding Sleeve, Torque, T5 Shank
I.T.S. GmbH
Orthopaedic implantation sleeve, reusable| Primary Device ID | 09120047306147 |
| NIH Device Record Key | 5dfd1ff9-eba2-4179-8a7a-ccef3601b991 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Sleeve |
| Version Model Number | 54101-80-2 |
| Catalog Number | 54101-80-2 |
| Company DUNS | 303456917 |
| Company Name | I.T.S. GmbH |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 09120047306147 [Primary] |
FDA Product Code
| LXH | Orthopedic Manual Surgical Instrument |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
[09120047306147]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2024-10-01 |
| Device Publish Date | 2018-09-19 |
On-Brand Devices [Sleeve]
| 09120047308370 | Sleeve, Eccentric |
| 09120047306246 | Measuring Sleeve, Measuring Length 120mm |
| 09120047306147 | Self Holding Sleeve, Torque, T5 Shank |
| 09120034309823 | Tissue Protection Sleeve, |
| 09120034303708 | Tissue Protection Sleeve, Can. 4.0mm Screw, 9mm Thread |
Trademark Results [Sleeve]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SLEEVE 98741417 not registered Live/Pending |
He Zan 2024-09-09 |
 SLEEVE 88507611 not registered Live/Pending |
Hvide, Johan Anders 2019-07-10 |
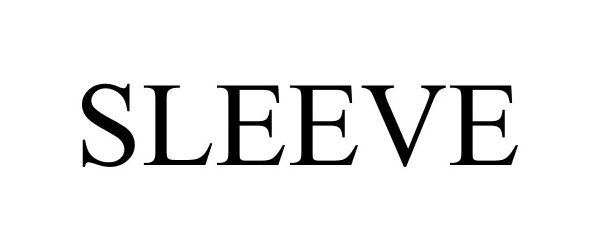 SLEEVE 87897863 not registered Dead/Abandoned |
Metaforge Apps Inc. 2018-04-27 |
 SLEEVE 78355144 not registered Dead/Abandoned |
Plasticade Products Corporation 2004-01-21 |
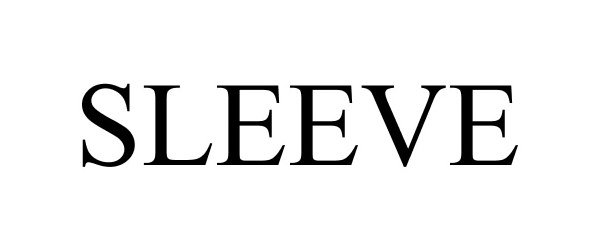 SLEEVE 77716932 not registered Dead/Abandoned |
Zuniga, Margaret Lynn 2009-04-18 |
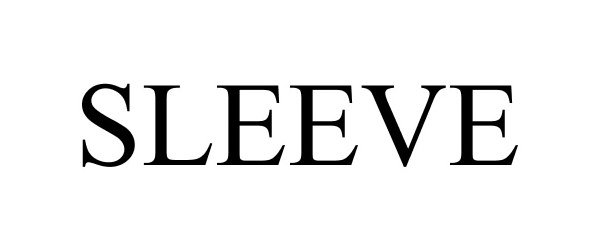 SLEEVE 77716932 not registered Dead/Abandoned |
Zuniga, Jesse 2009-04-18 |
 SLEEVE 74433883 not registered Dead/Abandoned |
Sony Corporation 1993-09-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.