TASK FORCE® MONITOR 3040i 00361
GUDID 09120073930644
Main Unit;
CNSystems Medizintechnik AG
Noninvasive haemodynamic monitoring system| Primary Device ID | 09120073930644 |
| NIH Device Record Key | 15b21945-7af2-4ad0-a70e-bc27c6296119 |
| Commercial Distribution Discontinuation | 2019-11-19 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | TASK FORCE® MONITOR 3040i |
| Version Model Number | V2 |
| Catalog Number | 00361 |
| Company DUNS | 303562672 |
| Company Name | CNSystems Medizintechnik AG |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +4331672345633 |
| service@cnsystems.com |
Operating and Storage Conditions
| Handling Environment Temperature | Between 10 Degrees Celsius and 40 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 09120073930644 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K014063
FDA Product Code
| DXN | System, Measurement, Blood-Pressure, Non-Invasive |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2019-11-20 |
| Device Publish Date | 2016-10-27 |
Devices Manufactured by CNSystems Medizintechnik AG
| 09120073930026 - AUX: Analog Out connector | 2019-11-20 Unit; connector for data analog output |
| 09120073930033 - BP Wave Out: CNAP® transducer cable | 2019-11-20 Unit; grey; adapter to interface cable |
| 09120073930040 - BP Wave Out: CNAP® transducer cable | 2019-11-20 Unit; black; adapter to interface cable |
| 09120073930057 - BP Wave Out: CNAP® transducer cable | 2019-11-20 Unit; red; adapter to interface cable |
| 09120073930064 - BP Wave Out: CNAP® transducer cable | 2019-11-20 Unit; yellow; adapter to interface cable |
| 09120073930095 - CNAP® cable | 2019-11-20 Unit; 2.5m; interface 09120073930774 CNAP® controller to 09120073930750 CNAP® Monitor 500 |
| 09120073930118 - CNAP® controller set | 2019-11-20 SET of: 09120073930101, CNAP® controller 09120073930088, CNAP® controller clamp |
| 09120073930125 - CNAP® double finger cuff "large" | 2019-11-20 Unit; sensor with Extended Lifecycle (150h) |
Trademark Results [TASK FORCE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
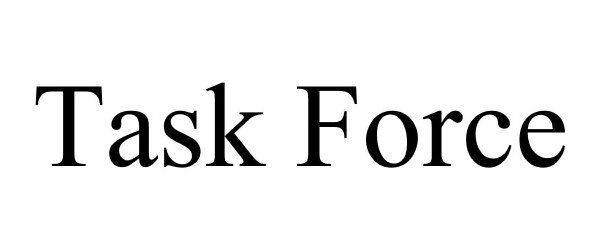 TASK FORCE 87778548 5698956 Live/Registered |
Xu, Rui P 2018-01-31 |
 TASK FORCE 78949302 not registered Dead/Abandoned |
LF, LLC 2006-08-10 |
 TASK FORCE 78690871 3732817 Dead/Cancelled |
LF, LLC 2005-08-11 |
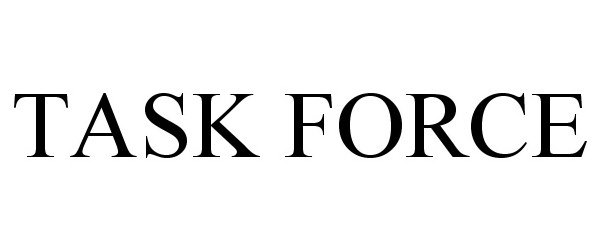 TASK FORCE 78666373 not registered Dead/Abandoned |
LF, LLC 2005-07-08 |
 TASK FORCE 78619366 not registered Dead/Abandoned |
LF, LLC 2005-04-28 |
 TASK FORCE 78555595 3581669 Dead/Cancelled |
LF, LLC 2005-01-28 |
 TASK FORCE 78505250 3092429 Dead/Expired |
LF, LLC 2004-10-25 |
 TASK FORCE 78480746 3674458 Live/Registered |
LF, LLC 2004-09-09 |
 TASK FORCE 78465640 3588937 Dead/Cancelled |
LF, LLC 2004-08-11 |
 TASK FORCE 78346580 not registered Dead/Abandoned |
LF, LLC 2003-12-30 |
 TASK FORCE 78337588 not registered Dead/Abandoned |
LF, LLC 2003-12-08 |
 TASK FORCE 78255518 not registered Dead/Abandoned |
LF, LLC 2003-05-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.