Sonex-HL
GUDID 09350855000092
Sonex-HL Each unit AHECC 2847.00.00 (NNA VARIANT)
NANOSONICS LIMITED
Medical device disinfection agent| Primary Device ID | 09350855000092 |
| NIH Device Record Key | d4d87aca-771b-4e12-a698-d05756652629 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Sonex-HL |
| Version Model Number | N00037-NNA |
| Company DUNS | 741021943 |
| Company Name | NANOSONICS LIMITED |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 09350855000030 [Primary] |
| GS1 | 09350855000085 [Package] Contains: 09350855000030 Package: [6 Units] In Commercial Distribution |
| GS1 | 09350855000092 [Package] Contains: 09350855000085 Package: [2 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K103059
FDA Product Code
| OUJ | High Level Disinfection Reprocessing Instrument For Ultrasonic Transducers |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2020-09-09 |
| Device Publish Date | 2016-09-21 |
On-Brand Devices [Sonex-HL]
| 09350855000078 | Sonex-HL Each Unit AHECC 2847.00.00 (GE VARIANT) |
| 09350855000092 | Sonex-HL Each unit AHECC 2847.00.00 (NNA VARIANT) |
Trademark Results [Sonex-HL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
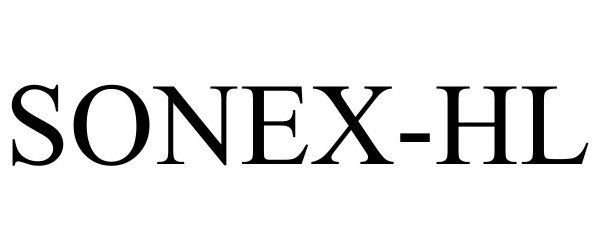 SONEX-HL 97765260 not registered Live/Pending |
Nanosonics Limited 2023-01-23 |
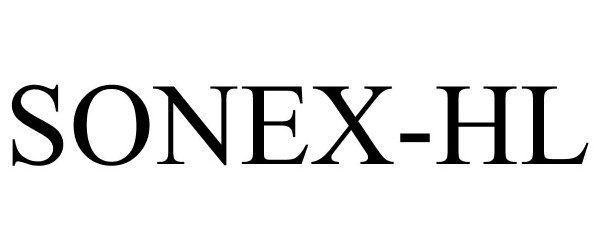 SONEX-HL 85190955 4168706 Live/Registered |
Nanosonics Limited 2010-12-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.