EasyScreen™ Gastrointestinal Parasite Detection Kit EP005-HT
GUDID 09351179001161
The EasyScreen™ Gastrointestinal Parasite Detection Kit is intended for use as an aid in the diagnosis of gastrointestinal illness by qualitative detection of Gastrointestinal Parasites from stool of patients with diarrhoea.
GENETIC SIGNATURES LIMITED
Multiple faecal parasite nucleic acid IVD, kit, nucleic acid technique (NAT)| Primary Device ID | 09351179001161 |
| NIH Device Record Key | 765c6820-4711-413d-bb34-8f7f9180b620 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | EasyScreen™ Gastrointestinal Parasite Detection Kit |
| Version Model Number | 1 |
| Catalog Number | EP005-HT |
| Company DUNS | 741249601 |
| Company Name | GENETIC SIGNATURES LIMITED |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +61298707580 |
| northamerica@geneticsignatures.com | |
| Phone | +61298707580 |
| northamerica@geneticsignatures.com | |
| Phone | +61298707580 |
| northamerica@geneticsignatures.com | |
| Phone | +61298707580 |
| northamerica@geneticsignatures.com | |
| Phone | +61298707580 |
| northamerica@geneticsignatures.com | |
| Phone | +61298707580 |
| northamerica@geneticsignatures.com | |
| Phone | +61298707580 |
| northamerica@geneticsignatures.com | |
| Phone | +61298707580 |
| northamerica@geneticsignatures.com | |
| Phone | +61298707580 |
| northamerica@geneticsignatures.com | |
| Phone | +61298707580 |
| northamerica@geneticsignatures.com | |
| Phone | +61298707580 |
| northamerica@geneticsignatures.com | |
| Phone | +61298707580 |
| northamerica@geneticsignatures.com | |
| Phone | +61298707580 |
| northamerica@geneticsignatures.com | |
| Phone | +61298707580 |
| northamerica@geneticsignatures.com |
Operating and Storage Conditions
| Storage Environment Temperature | Between -25 Degrees Celsius and -15 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -15 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -15 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -15 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -15 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -15 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -15 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -15 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -15 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -15 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -15 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -15 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -15 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -15 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 09351179001161 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K232672
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-06-26 |
| Device Publish Date | 2024-06-18 |
Devices Manufactured by GENETIC SIGNATURES LIMITED
| 09351179000225 - EasyScreen™ Sample Processing Kit | 2024-12-11 The EasyScreen™ Sample Processing Kit (SP001) is a kit that contains reagents to isolate, sodium bisulphite treat and purify n |
| 09351179000003 - EasyScreen™ Sample Processing Kit | 2024-11-26 The EasyScreen™ Sample Processing Kit (SP012) is designed to rapidly isolate nucleic acids (DNA and RNA) from clinical samples |
| 09351179000188 - EasyScreen™ Sample Processing Kit | 2024-06-26 The EasyScreen™ Sample Processing Kit (SP008B) is designed to rapidly isolate nucleic acids (DNA and RNA) from clinical sample |
| 09351179001161 - EasyScreen™ Gastrointestinal Parasite Detection Kit | 2024-06-26The EasyScreen™ Gastrointestinal Parasite Detection Kit is intended for use as an aid in the diagnosis of gastrointestinal illness by qualitative detection of Gastrointestinal Parasites from stool of patients with diarrhoea. |
| 09351179001161 - EasyScreen™ Gastrointestinal Parasite Detection Kit | 2024-06-26 The EasyScreen™ Gastrointestinal Parasite Detection Kit is intended for use as an aid in the diagnosis of gastrointestinal ill |
| 09351179004070 - EasyScreen™ Gastrointestinal Parasite PCR Positive Control | 2024-06-26 The EasyScreen™ Gastrointestinal Parasite PCR Positive Control (PC-PROT-001) is a control material for nucleic acid testing of |
Trademark Results [EasyScreen]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
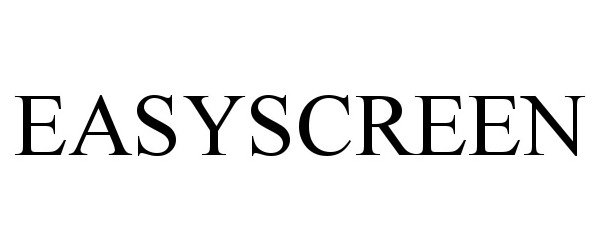 EASYSCREEN 98505769 not registered Live/Pending |
Sunset Products Limited Partnership 2024-04-17 |
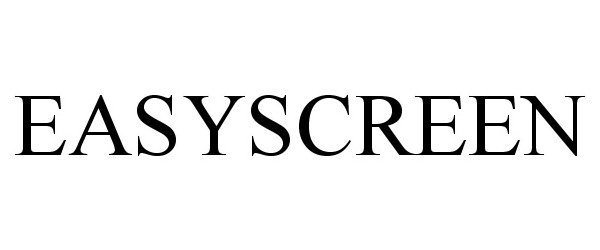 EASYSCREEN 85537293 4639209 Live/Registered |
Callum Bush 2012-02-08 |
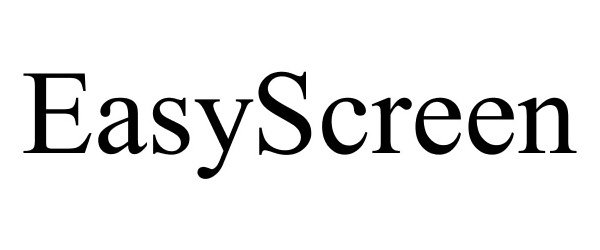 EASYSCREEN 85325453 4211013 Live/Registered |
Nuvasive Inc. 2011-05-19 |
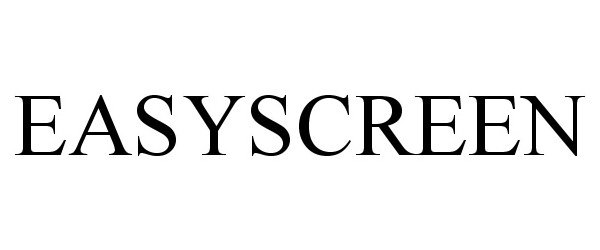 EASYSCREEN 85163837 4281345 Dead/Cancelled |
MAREX SPECTRON GROUP LIMITED 2010-10-28 |
 EASYSCREEN 79391120 not registered Live/Pending |
Genetic Signatures Limited 2024-02-14 |
 EASYSCREEN 79345602 not registered Live/Pending |
Genetic Signatures Limited 2021-08-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.