Tabu 70011
GUDID 10060009539113
Natural Personal Lubricant - 100 ml
MAYER LABORATORIES, INC.
Sexual lubricant| Primary Device ID | 10060009539113 |
| NIH Device Record Key | 9d7039d6-73cd-4aba-8b5f-dddcaae12210 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Tabu |
| Version Model Number | Tabu Natural Lubricant |
| Catalog Number | 70011 |
| Company DUNS | 175425982 |
| Company Name | MAYER LABORATORIES, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com | |
| Phone | +1(510)229-5300 |
| davidm@mayerlabs.com |
Device Dimensions
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
| Total Volume | 100 Milliliter |
Operating and Storage Conditions
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
| Storage Environment Temperature | Between 40 Degrees Fahrenheit and 90 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *NO DIRECT SUNLIGHT OR HEAT |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00060009539116 [Primary] |
| GS1 | 10060009539113 [Package] Contains: 20060009539110 Package: MASTER CARTON [6 Units] In Commercial Distribution |
| GS1 | 20060009539110 [Package] Contains: 00060009539116 Package: FOLDED CARTON [6 Units] In Commercial Distribution |
FDA Product Code
| NUC | Lubricant, personal |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-11-13 |
| Device Publish Date | 2023-11-03 |
On-Brand Devices [Tabu]
| 10060009539113 | Natural Personal Lubricant - 100 ml |
| 10060009539106 | Natural Personal Lubricant - 50 ml |
Trademark Results [Tabu]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
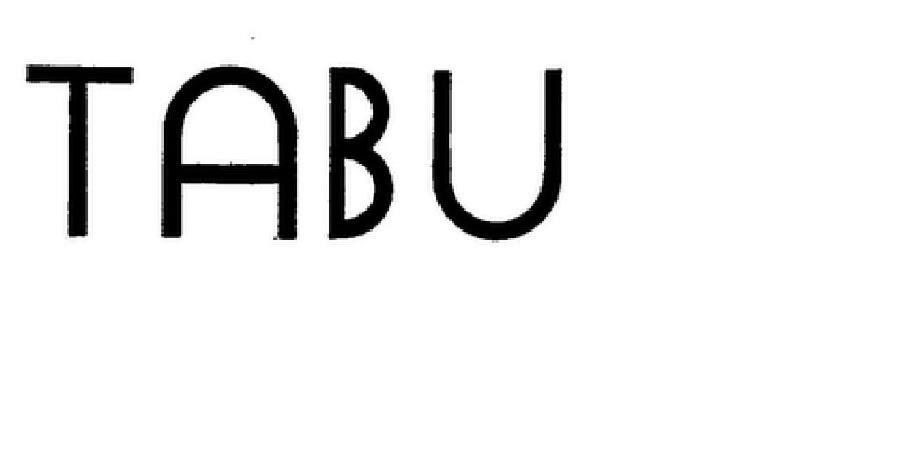 TABU 98356881 not registered Live/Pending |
FRAGRANCE XTREME, INC. 2024-01-14 |
 TABU 98224451 not registered Live/Pending |
Theodore Oakley 2023-10-15 |
 TABU 97781627 not registered Live/Pending |
Tao Systems LLC 2023-02-06 |
 TABU 97223913 not registered Live/Pending |
WORLD OF LOTUS LLC 2022-01-18 |
 TABU 90727822 not registered Live/Pending |
Fiendish Spirits 2021-05-21 |
 TABU 90400869 not registered Live/Pending |
Zhao,Qiong 2020-12-22 |
 TABU 90189365 not registered Live/Pending |
Tabu Group Inc. 2020-09-17 |
 TABU 87514640 not registered Dead/Abandoned |
Sliuman, Rita 2017-07-03 |
 TABU 87455536 not registered Dead/Abandoned |
BODEGA LUIGI BOSCA E HIJOS S.R.L. 2017-05-18 |
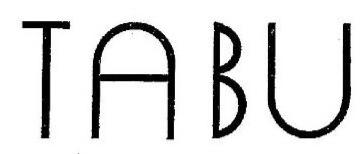 TABU 85865865 4414534 Live/Registered |
Finanz St. Honore, B.V. 2013-03-04 |
 TABU 85464657 4174707 Dead/Cancelled |
Cooks, Kelvin 2011-11-04 |
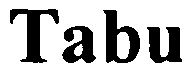 TABU 79084649 3896666 Live/Registered |
Felix Rauter GmbH & Co. KG 2010-05-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.