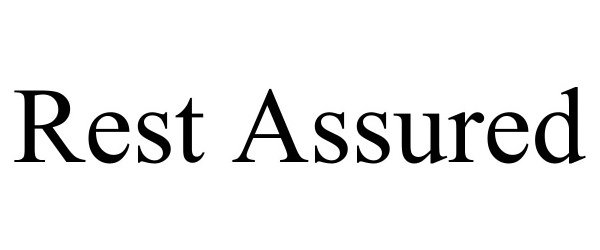Rest Assured
GUDID 10071031189528
Snore No More 89325AA
RANIR, LLC
Mandible-repositioning sleep-disordered breathing orthosis| Primary Device ID | 10071031189528 |
| NIH Device Record Key | 5d1fc80a-f3aa-4ad9-8d54-bc7bfe1f29ca |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Rest Assured |
| Version Model Number | ZWN |
| Company DUNS | 364567615 |
| Company Name | RANIR, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00071031189521 [Primary] |
| GS1 | 10071031189528 [Package] Contains: 00071031189521 Package: INNER [3 Units] In Commercial Distribution |
| GS1 | 20071031189525 [Package] Package: SHIPPER [12 Units] In Commercial Distribution |
FDA Product Code
| LRK | Device, Anti-Snoring |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-10-25 |
| Device Publish Date | 2021-10-15 |
On-Brand Devices [Rest Assured]
| 20651080000004 | SNORE NO MORE 1CT |
| 10071031189528 | Snore No More 89325AA |
Trademark Results [Rest Assured]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.