Compound W®
GUDID 10075137530055
Maximum Freeze Freeze Off® Wart Remover System
MEDTECH PRODUCTS INC.
Wart-removal cryogenic kit| Primary Device ID | 10075137530055 |
| NIH Device Record Key | c918b489-5848-4112-be61-6f55a060a30a |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Compound W® |
| Version Model Number | CW017201, CW017105 |
| Company DUNS | 060272465 |
| Company Name | MEDTECH PRODUCTS INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx | |
| Phone | 1-800-443-4908 |
| xx@xx.xx |
Operating and Storage Conditions
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 120 Degrees Fahrenheit |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00075137530058 [Primary] |
| GS1 | 10075137530055 [Package] Contains: 00075137530058 Package: Shipping case [12 Units] In Commercial Distribution |
FDA Product Code
| GEH | Unit, Cryosurgical, Accessories |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2018-03-29 |
| Device Publish Date | 2016-09-16 |
On-Brand Devices [Compound W®]
| 10075137530055 | Maximum Freeze Freeze Off® Wart Remover System |
| 10075137440347 | Maximum Freeze Freeze Off® Plantar Kit Wart Removal System |
| 00075137110854 | COMPOUND W® DUAL POWER (2-in-1 Treatment Kit for Large Warts) |
| 00075137110571 | COMPOUND W® COMPLETE WART KIT Maximum Freeze Plantar and Stubborn Warts |
| 00075137108257 | Accu-Freeze® Freeze Off® Advanced Wart Removal System |
| 00075137000032 | Compound W® Nitrofreeze™ Nitrous Oxide Wart Removal System |
| 10075137000145 | Compound W® Nitrofreeze™ Nitrous Oxide Wart Removal System - yellow carton secondary sku |
Trademark Results [Compound W]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COMPOUND W 86346153 4695707 Live/Registered |
Medtech Products Inc. 2014-07-23 |
 COMPOUND W 85049641 4235884 Live/Registered |
Medtech Products Inc. 2010-05-27 |
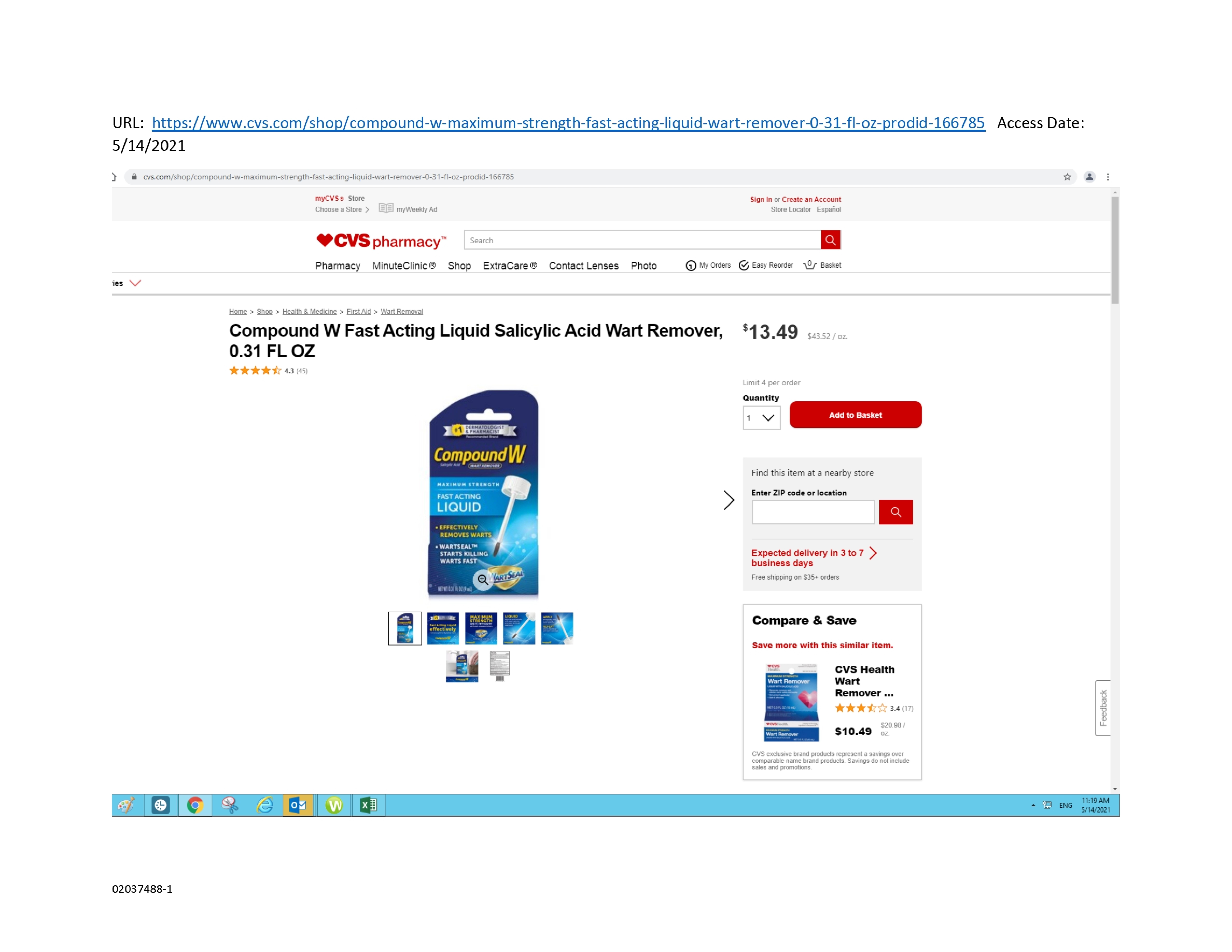 COMPOUND W 72104800 0716021 Live/Registered |
American Home Products Corporation 1960-09-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.