CORE VALUES
GUDID 10079573108519
HARMON ANTI-NAUSEA WRIST BAND 12/144
APOTHECARY PRODUCTS, LLC
Acupressure wristband| Primary Device ID | 10079573108519 |
| NIH Device Record Key | 4577c737-0048-4ce1-9538-0acf57d83ffd |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | CORE VALUES |
| Version Model Number | 400851 |
| Company DUNS | 092312735 |
| Company Name | APOTHECARY PRODUCTS, LLC |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | 952-890-1940 |
| UDI@APOTHECARYPRODUCTS.COM | |
| Phone | 952-890-1940 |
| UDI@APOTHECARYPRODUCTS.COM | |
| Phone | 952-890-1940 |
| UDI@APOTHECARYPRODUCTS.COM | |
| Phone | 952-890-1940 |
| UDI@APOTHECARYPRODUCTS.COM | |
| Phone | 952-890-1940 |
| UDI@APOTHECARYPRODUCTS.COM | |
| Phone | 952-890-1940 |
| UDI@APOTHECARYPRODUCTS.COM | |
| Phone | 952-890-1940 |
| UDI@APOTHECARYPRODUCTS.COM | |
| Phone | 952-890-1940 |
| UDI@APOTHECARYPRODUCTS.COM | |
| Phone | 952-890-1940 |
| UDI@APOTHECARYPRODUCTS.COM | |
| Phone | 952-890-1940 |
| UDI@APOTHECARYPRODUCTS.COM | |
| Phone | 952-890-1940 |
| UDI@APOTHECARYPRODUCTS.COM | |
| Phone | 952-890-1940 |
| UDI@APOTHECARYPRODUCTS.COM | |
| Phone | 952-890-1940 |
| UDI@APOTHECARYPRODUCTS.COM |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00079573108512 [Primary] |
| GS1 | 10079573108519 [Package] Contains: 00079573108512 Package: inner [12 Units] In Commercial Distribution |
| GS1 | 20079573108516 [Package] Package: case [12 Units] In Commercial Distribution |
FDA Product Code
| MVV | Device, Acupressure |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-09-08 |
| Device Publish Date | 2020-08-31 |
On-Brand Devices [CORE VALUES]
| 10079573108564 | HARMON PILL SPLITTER 6/144 |
| 10079573108519 | HARMON ANTI-NAUSEA WRIST BAND 12/144 |
| 10079573108304 | HARMON INSTANT COLD COMPRESS (1PK) 24/48 |
Trademark Results [CORE VALUES]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CORE VALUES 98859500 not registered Live/Pending |
Smiling Tree Toys LLC 2024-11-18 |
 CORE VALUES 87319208 not registered Dead/Abandoned |
Core Values Apparel 2017-01-31 |
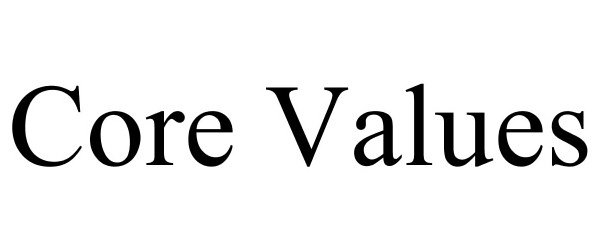 CORE VALUES 86892319 5312206 Live/Registered |
4 Hands Brewing Company, LLC 2016-01-31 |
 CORE VALUES 86855330 5781958 Live/Registered |
Harmon Stores, Inc. 2015-12-21 |
 CORE VALUES 86675740 5101254 Live/Registered |
Robert Charles Albert III 2015-06-26 |
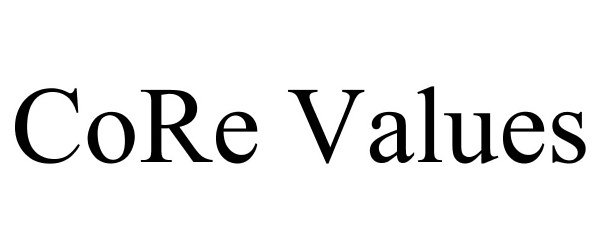 CORE VALUES 85196453 not registered Dead/Abandoned |
Home Entertainment Distributors, Inc. 2010-12-13 |
 CORE VALUES 85143505 not registered Dead/Abandoned |
Smith Asset Management Group, LP 2010-10-01 |
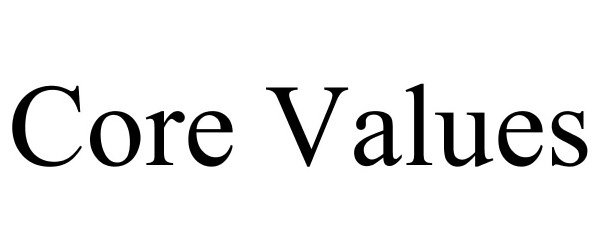 CORE VALUES 78671891 3495986 Dead/Cancelled |
PugMedia Inc. 2005-07-16 |
 CORE VALUES 78357514 2970247 Dead/Cancelled |
Summer Industries, LLC 2004-01-26 |
 CORE VALUES 77847704 3915643 Dead/Cancelled |
Core, Chris 2009-10-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.