ISOCOOL® 8145025S
GUDID 10381780517344
AHT Bipolar Forceps Tips 9 1/2 Inches (24.1cm) Total Length 0.25mm Tip Diameter 4 1/2 Inches (11.4cm) Working Length TIP STYLE Low Profile
INTEGRA LIFESCIENCES PRODUCTION CORPORATION
Open-surgery electrosurgical handpiece/electrode, bipolar, reusable| Primary Device ID | 10381780517344 |
| NIH Device Record Key | 602462e2-9e31-4652-b2d5-b136fc52b831 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | ISOCOOL® |
| Version Model Number | 8145025S |
| Catalog Number | 8145025S |
| Company DUNS | 081277700 |
| Company Name | INTEGRA LIFESCIENCES PRODUCTION CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com | |
| Phone | +1(800)654-2873 |
| custsvcnj@integralife.com |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 24.1 Centimeter |
| Length | 11.4 Centimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10381780517344 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K083895
FDA Product Code
| GEI | Electrosurgical, cutting & coagulation & accessories |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2020-05-11 |
| Device Publish Date | 2020-04-01 |
On-Brand Devices [ISOCOOL®]
| 10381780519546 | AHT Bipolar Forceps Tips 8 1/2 Inches (22cm) Total Length 1mm Tip diameter 3 1/2 Inches (8.9cm) |
| 10381780517412 | AHT Bipolar Forceps Tips 10 1/2 Inches (26.7cm) Total Length 2mm Tip Diameter 5 1/2 Inches (14cm |
| 10381780517405 | AHT Bipolar Forceps Tips 10 1/2 Inches (26.7cm) Total Length 1mm Tip diameter 5 1/2 Inches (14cm |
| 10381780517399 | AHT Bipolar Forceps Tips 10 1/2 Inches (26.7cm) Total Length 0.5mm Tip Diameter 5 1/2 Inches (14 |
| 10381780517382 | AHT Bipolar Forceps Tips 10 1/2 Inches (26.7cm) Total Length 0.25mm Tip Diameter 5 1/2 Inches (1 |
| 10381780517375 | AHT Bipolar Forceps Tips 9 1/2 Inches (24.1cm) Total Length 2mm Tip Diameter 4 1/2 Inches (11.4c |
| 10381780517368 | AHT Bipolar Forceps Tips 9 1/2 Inches (24.1cm) Total Length 1mm Tip diameter 4 1/2 Inches (11.4c |
| 10381780517351 | AHT Bipolar Forceps Tips 9 1/2 Inches (24.1cm) Total Length 0.5mm Tip Diameter 4 1/2 Inches (11. |
| 10381780517344 | AHT Bipolar Forceps Tips 9 1/2 Inches (24.1cm) Total Length 0.25mm Tip Diameter 4 1/2 Inches (11 |
| 10381780517337 | AHT Bipolar Forceps Tips 8 1/2 Inches (22cm) Total Length 2mm Tip Diameter 3 1/2 Inches (8.9cm) |
| 10381780517320 | AHT Bipolar Forceps Tips 8 1/2 Inches (22cm) Total Length 1mm Tip diameter 3 1/2 Inches (8.9cm) |
| 10381780517313 | AHT Bipolar Forceps Tips 8 1/2 Inches (22cm) Total Length 0.5mm Tip Diameter 3 1/2 Inches (8.9cm |
| 10381780517306 | AHT Bipolar Forceps Tips 8 1/2 Inches (22cm) Total Length 0.25mm Tip Diameter 3 1/2 Inches (8.9c |
Trademark Results [ISOCOOL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
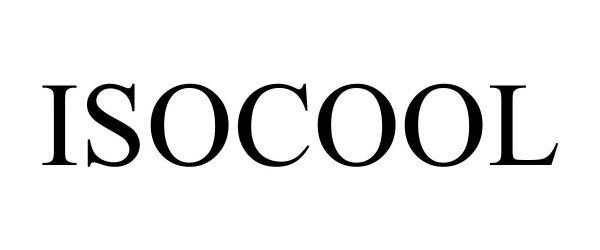 ISOCOOL 97562025 not registered Live/Pending |
Mountain Warehouse Limited 2022-08-24 |
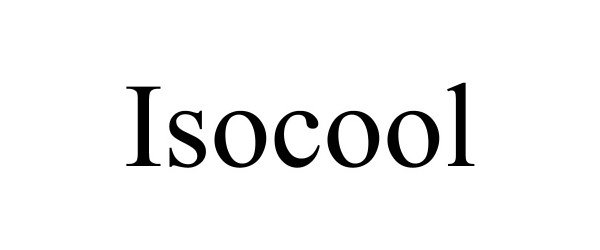 ISOCOOL 90250331 not registered Live/Pending |
Hu Hongjun 2020-10-13 |
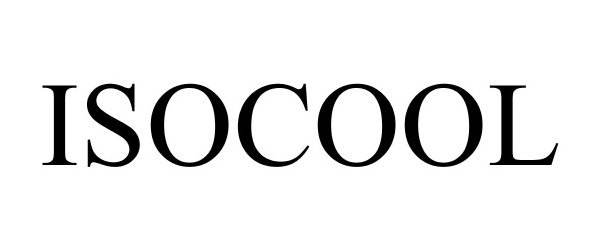 ISOCOOL 86295820 4806534 Live/Registered |
Mountain Warehouse Limited 2014-05-30 |
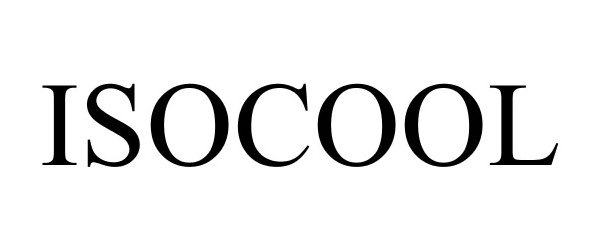 ISOCOOL 85150974 3970624 Live/Registered |
INTEGRA LIFESCIENCES CORPORATION 2010-10-12 |
 ISOCOOL 85002127 3975682 Dead/Cancelled |
Purcell Systems, Inc. 2010-03-30 |
 ISOCOOL 78491118 3202672 Live/Registered |
Ultimate Nutrition, Inc. 2004-09-28 |
 ISOCOOL 78098119 2803565 Dead/Cancelled |
JOHNSON & JOHNSON 2001-12-13 |
 ISOCOOL 74352588 not registered Dead/Abandoned |
PETROFER Chemie H.R. Fischer GmbH & Co.KG 1993-05-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.