S-ROM 522030
GUDID 10603295170815
S-ROM FEMORAL HEAD 11/13 TAPER Diameter 28mm +12
DEPUY INTERNATIONAL LTD
Metallic femoral head prosthesis| Primary Device ID | 10603295170815 |
| NIH Device Record Key | 80a41c2d-68b4-43a8-bf44-6dd085daf65f |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | S-ROM |
| Version Model Number | 52-2030 |
| Catalog Number | 522030 |
| Company DUNS | 896498813 |
| Company Name | DEPUY INTERNATIONAL LTD |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx | |
| Phone | +1(800)255-2500 |
| xx@xx.xx |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10603295170815 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K920317
FDA Product Code
| JDI | Prosthesis, hip, semi-constrained, metal/polymer, cemented |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2020-04-06 |
| Device Publish Date | 2015-09-24 |
On-Brand Devices [S-ROM]
| 10603295219712 | S-ROM A STEM IMPACTOR/INSERTER |
| 10603295218869 | S-ROM FEMORAL HEAD 11/13 TAPER Diameter 32mm +9 |
| 10603295218852 | S-ROM FEMORAL HEAD 11/13 TAPER Diameter 32mm +3 |
| 10603295218845 | S-ROM FEMORAL HEAD 11/13 TAPER Diameter 28mm +9 |
| 10603295218838 | S-ROM FEMORAL HEAD 11/13 TAPER Diameter 28mm +3 |
| 10603295170846 | S-ROM FEMORAL HEAD 11/13 TAPER Diameter 32mm +12 |
| 10603295170839 | S-ROM FEMORAL HEAD 11/13 TAPER Diameter 32mm +6 |
| 10603295170822 | S-ROM FEMORAL HEAD 11/13 TAPER Diameter 32mm +0 |
| 10603295170815 | S-ROM FEMORAL HEAD 11/13 TAPER Diameter 28mm +12 |
| 10603295170808 | S-ROM FEMORAL HEAD 11/13 TAPER Diameter 28mm +6 |
| 10603295170792 | S-ROM FEMORAL HEAD 11/13 TAPER Diameter 28mm +0 |
| 10603295170778 | S-ROM FEMORAL HEAD 11/13 TAPER Diameter 22.225mm +0 |
| 10603295033141 | S-ROM M METAL ON METAL FEMORAL HEAD Diameter 28mm +0 12/14 TAPER ULTAMET |
Trademark Results [S-ROM]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
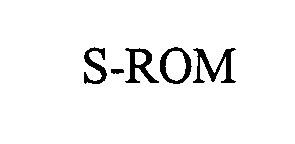 S-ROM 76516840 not registered Dead/Abandoned |
JOHNSON & JOHNSON 2003-05-01 |
 S-ROM 74087580 1701594 Live/Registered |
JOHNSON & JOHNSON 1990-08-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.