Alexis® O Wound Protector-Retractor
GUDID 10607915140325
Wound Retractor and Protector
APPLIED MEDICAL RESOURCES CORPORATION
Radial surgical retractor| Primary Device ID | 10607915140325 |
| NIH Device Record Key | fba3450c-a57f-42d2-a337-86ba24a15e1e |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Alexis® O Wound Protector-Retractor |
| Version Model Number | C8406 |
| Company DUNS | 187129135 |
| Company Name | APPLIED MEDICAL RESOURCES CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00607915140328 [Primary] |
| GS1 | 10607915140325 [Package] Contains: 00607915140328 Package: [3 Units] In Commercial Distribution |
FDA Product Code
| KGW | Ring (Wound Protector), Drape Retention, Internal |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2024-09-04 |
| Device Publish Date | 2022-05-27 |
Devices Manufactured by APPLIED MEDICAL RESOURCES CORPORATION
| 00607915146467 - Voyant Electrosurgical Generator | 2026-01-26 |
| 00607915149024 - Inzii Ripstop Retrieval System | 2026-01-26 |
| 00607915140700 - Alexis® Orthopaedic Protector | 2025-04-16 X-S/M Flexible Alexis Orthopaedic Protector |
| 00607915114688 - Threaded Cannula and Seal | 2024-12-16 Laparoscopic Trocar Sleeve |
| 10607915140325 - Alexis® O Wound Protector-Retractor | 2024-09-04Wound Retractor and Protector |
| 10607915140325 - Alexis® O Wound Protector-Retractor | 2024-09-04 Wound Retractor and Protector |
| 10607915145528 - Inzii Ripstop Redeployable Retrieval System | 2024-03-27 Redeployable tissue retrieval system with Ripstop nylon bag. |
| 00607915146498 - Suction Irrigation System | 2024-02-09 Probe and Valve with dual bag tubing. |
| 00607915146504 - Suction Irrigation System | 2024-02-09 Valve with dual bag tubing. |
Trademark Results [Alexis]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
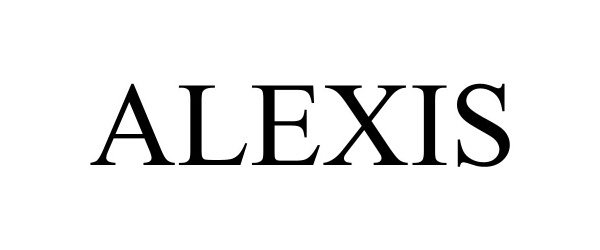 ALEXIS 98115554 not registered Live/Pending |
PRODUCE IN PARADISE, INC. 2023-08-03 |
 ALEXIS 98115547 not registered Live/Pending |
PRODUCE IN PARADISE, INC. 2023-08-03 |
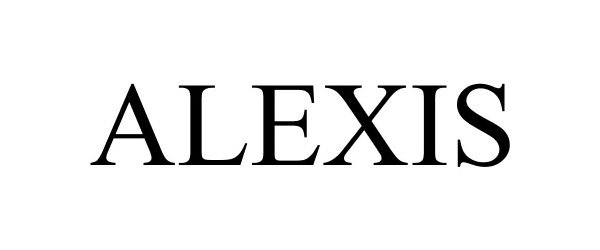 ALEXIS 88582189 not registered Live/Pending |
Sound Seal, Inc. 2019-08-16 |
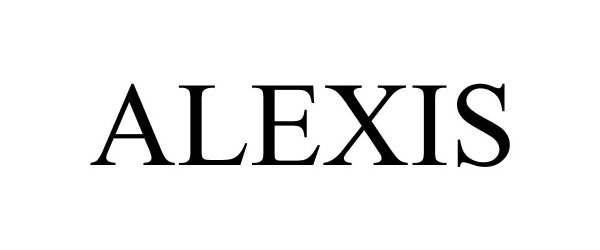 ALEXIS 88476089 5949699 Live/Registered |
Freeman, Alexis 2019-06-17 |
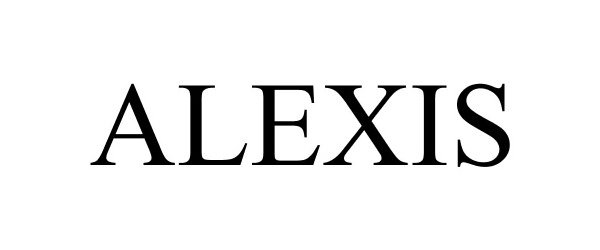 ALEXIS 88368118 not registered Live/Pending |
Sazerac Brands, LLC 2019-04-02 |
 ALEXIS 88190519 5780536 Live/Registered |
Alexis Fire Equipment Co. 2018-11-12 |
 ALEXIS 88094055 not registered Live/Pending |
Alexis, LLC 2018-08-27 |
 ALEXIS 88091208 not registered Live/Pending |
Alexis, LLC 2018-08-24 |
 ALEXIS 86885061 5131622 Live/Registered |
Spin Master Ltd. 2016-01-25 |
 ALEXIS 86542586 4844546 Live/Registered |
Applied Medical Resources Corporation 2015-02-23 |
 ALEXIS 85364806 not registered Dead/Abandoned |
Rapiscan Systems, Inc. 2011-07-06 |
 ALEXIS 79007542 not registered Dead/Abandoned |
Richard Lewis Eddy 2004-07-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.