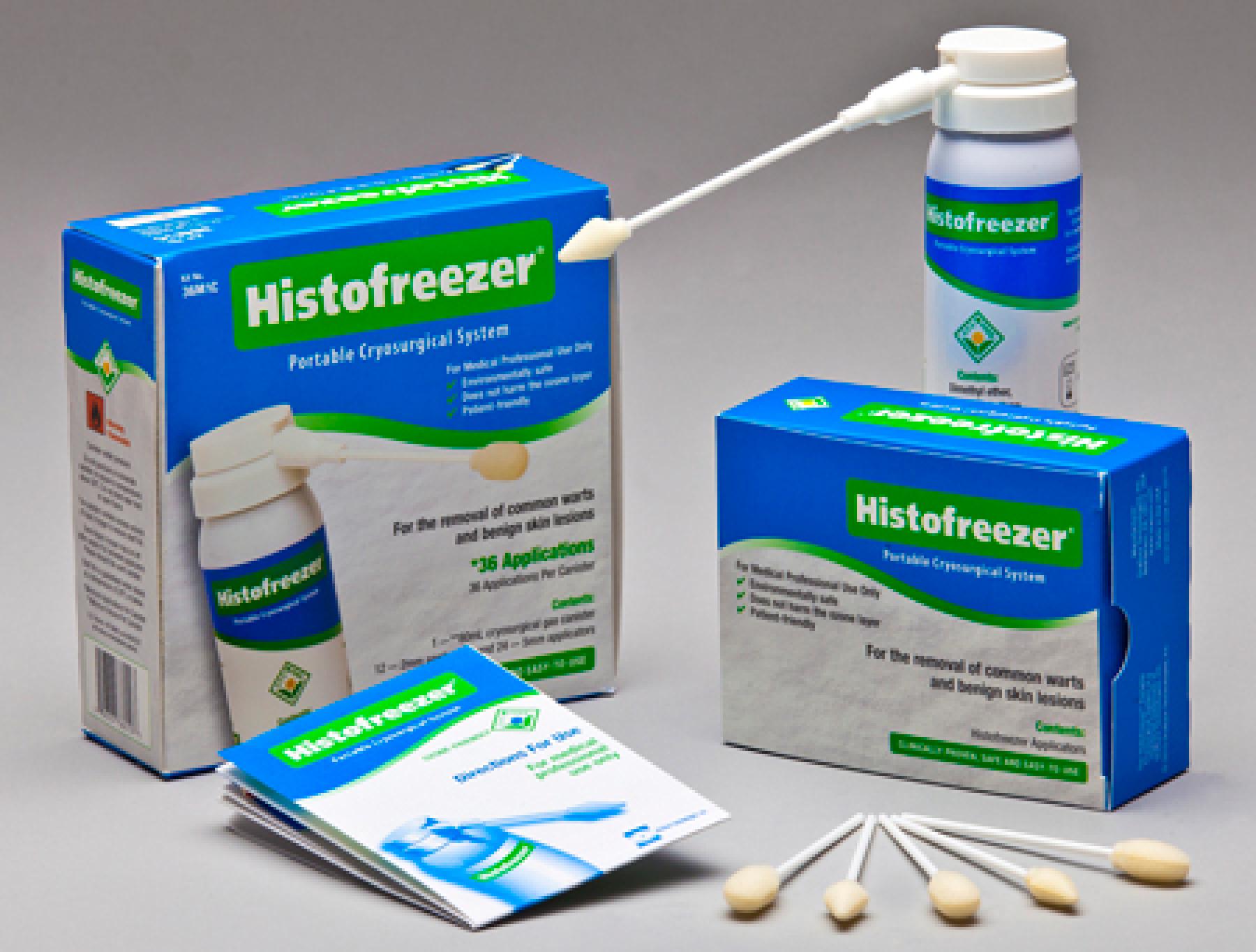
Histofreezer
GUDID 10608337365150
Histofreezer, Kit, U.S., 36F1C
Orasure Technologies, Inc.
General cryosurgical system, cryogen gas, mechanical| Primary Device ID | 10608337365150 |
| NIH Device Record Key | 9b0e3f41-2095-4a85-8040-0054f1a88c9b |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Histofreezer |
| Version Model Number | 1001-0294 |
| Company DUNS | 114152184 |
| Company Name | Orasure Technologies, Inc. |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com | |
| Phone | 610-882-1820 |
| customercare@orasure.com |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Storage Environment Temperature | Between 0 Degrees Celsius and 50 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Storage Environment Temperature | Between 0 Degrees Celsius and 50 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Storage Environment Temperature | Between 0 Degrees Celsius and 50 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Storage Environment Temperature | Between 0 Degrees Celsius and 50 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Storage Environment Temperature | Between 0 Degrees Celsius and 50 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Storage Environment Temperature | Between 0 Degrees Celsius and 50 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Storage Environment Temperature | Between 0 Degrees Celsius and 50 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Storage Environment Temperature | Between 0 Degrees Celsius and 50 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Storage Environment Temperature | Between 0 Degrees Celsius and 50 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Storage Environment Temperature | Between 0 Degrees Celsius and 50 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Storage Environment Temperature | Between 0 Degrees Celsius and 50 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Storage Environment Temperature | Between 0 Degrees Celsius and 50 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Storage Environment Temperature | Between 0 Degrees Celsius and 50 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Storage Environment Temperature | Between 0 Degrees Celsius and 50 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
| Storage Environment Temperature | Between 0 Degrees Celsius and 50 Degrees Celsius |
| Special Storage Condition, Specify | Between 0 and 0 *Protect from sunlight |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00608337365108 [Primary] |
| GS1 | 10608337365150 [Package] Contains: 00608337365108 Package: [5 Units] In Commercial Distribution |
FDA Product Code
| GEH | Unit, Cryosurgical, Accessories |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2018-03-29 |
| Device Publish Date | 2016-09-15 |
On-Brand Devices [Histofreezer]
| 10608337402152 | Histofreezer Kit, U.S., 40T1C |
| 10608337365150 | Histofreezer, Kit, U.S., 36F1C |
| 10608337360155 | Histofreezer Kit, U.S., 36M1C |
| 00608337001297 | Histofreezer, US Sample, 5M1C |
| 10608337000556 | Histofreezer Kit, U.S., 60MC2 +10 |
Trademark Results [Histofreezer]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HISTOFREEZER 73741009 1525479 Live/Registered |
KONINKLIJKE UTERMOHLEN N.V. 1988-07-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.