MECRON® Standard Tape DST-12
GUDID 10609271619996
White
DARCO INTERNATIONAL, INC.
Limb immobilization/support skin adhesive tape| Primary Device ID | 10609271619996 |
| NIH Device Record Key | a588e005-02b3-4395-94cf-8623217bc134 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | MECRON® Standard Tape |
| Version Model Number | DST-12 |
| Catalog Number | DST-12 |
| Company DUNS | 184231298 |
| Company Name | DARCO INTERNATIONAL, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00609271619999 [Primary] |
| GS1 | 10609271619996 [Package] Contains: 00609271619999 Package: Box [12 Units] In Commercial Distribution |
FDA Product Code
| KGX | Tape and bandage, adhesive |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-02-22 |
| Device Publish Date | 2023-02-14 |
Devices Manufactured by DARCO INTERNATIONAL, INC.
| 20609271989607 - Aircore™ Insole | 2026-01-15 Peg Off-Loading Insole for POGO® (Men's 4.5 / Women's 6.0) |
| 20609271989614 - Aircore™ Insole | 2026-01-15 Peg Off-Loading Insole for POGO® (Men's 5.5 / Women's 7.0) |
| 20609271989621 - Aircore™ Insole | 2026-01-15 Peg Off-Loading Insole for POGO® (Men's 6.0 / Women's 7.5 ) |
| 20609271989638 - Aircore™ Insole | 2026-01-15 Peg Off-Loading Insole for POGO® (Men's 6.5 / Women's 8.0) |
| 00609271989641 - Aircore™ Insole | 2026-01-15 Peg Off-Loading Insole for POGO® (Men's 7.0 / Women's 8.5 ) |
| 20609271989652 - Aircore™ Insole | 2026-01-15 Peg Off-Loading Insole for POGO® (Men's 7.5 / Women's 9.0) |
| 20609271989669 - Aircore™ Insole | 2026-01-15 Peg Off-Loading Insole for POGO® (Men's 8.0 / Women's 9.5 ) |
| 20609271989676 - Aircore™ Insole | 2026-01-15 Peg Off-Loading Insole for POGO® (Men's 8.5 / Women's 10 ) |
Trademark Results [MECRON]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
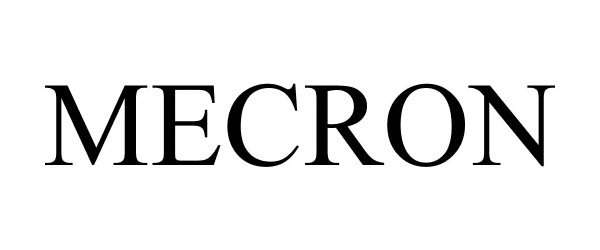 MECRON 87622435 5542558 Live/Registered |
Aristotech Holding GmbH 2017-09-26 |
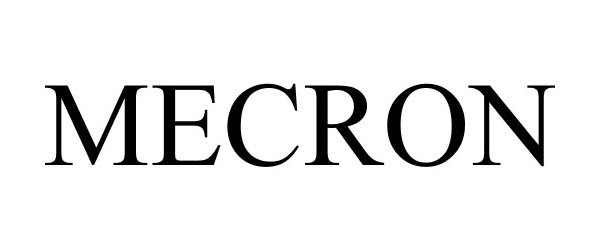 MECRON 86770769 5281806 Live/Registered |
DARCO INTERNATIONAL, INC. 2015-09-28 |
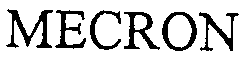 MECRON 76476396 not registered Indifferent |
MERETE MEDICAL GmbH 0000-00-00 |
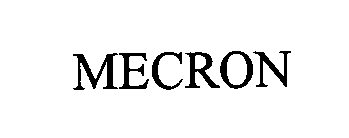 MECRON 76371126 2864071 Dead/Cancelled |
MERETE MEDICAL, INC. 2002-02-14 |
 MECRON 73223391 1161771 Dead/Cancelled |
Mecron Medizinische Produkte GmbH 1979-07-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.