Tri-Valent™ Swab CV-01
GUDID 10634853001364
Vaginitis Calibration Panel
MICROBIOLOGICS INC.
Multiple-type vaginitis-associated organism nucleic acid IVD, calibrator| Primary Device ID | 10634853001364 |
| NIH Device Record Key | 78747169-fdfd-4b92-a10b-451c7f675c2d |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Tri-Valent™ Swab |
| Version Model Number | CV-01 |
| Catalog Number | CV-01 |
| Company DUNS | 060467826 |
| Company Name | MICROBIOLOGICS INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
Operating and Storage Conditions
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10634853001364 [Primary] |
FDA Product Code
| JIT | Calibrator, secondary |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-10-04 |
| Device Publish Date | 2022-09-26 |
On-Brand Devices [Tri-Valent™ Swab]
| 10634853001418 | Vaginitis Validation Panel |
| 10634853001401 | Trichomonas Verification Set |
| 10634853001395 | Tri-Valent Negative Control Swab |
| 10634853001388 | Tri-Valent Positive Control Swab |
| 10634853001364 | Vaginitis Calibration Panel |
Trademark Results [Tri-Valent]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
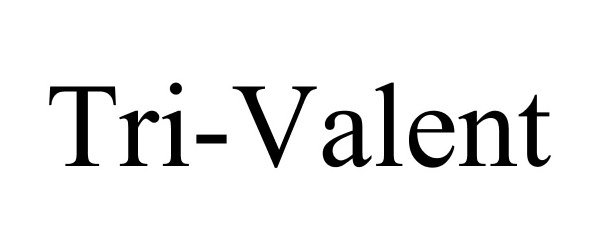 TRI-VALENT 77215603 3388337 Live/Registered |
GIBSON LABORATORIES LLC 2007-06-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.