Exacto 00711094
GUDID 10724995182554
The Exacto cold snare is intended to be used without diathermic energy for the endoscopic resection of polyp tissue in the gastrointestinal tract.
US Endoscopy
Mechanical-cutting endoscopic polypectomy snare| Primary Device ID | 10724995182554 |
| NIH Device Record Key | ce173739-adb8-4c03-bf0a-a7f6685bbba7 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Exacto |
| Version Model Number | 00711094 |
| Catalog Number | 00711094 |
| Company DUNS | 627879687 |
| Company Name | US Endoscopy |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)548-4873 |
| xx@xx.xx | |
| Phone | +1(800)548-4873 |
| xx@xx.xx | |
| Phone | +1(800)548-4873 |
| xx@xx.xx | |
| Phone | +1(800)548-4873 |
| xx@xx.xx | |
| Phone | +1(800)548-4873 |
| xx@xx.xx | |
| Phone | +1(800)548-4873 |
| xx@xx.xx | |
| Phone | +1(800)548-4873 |
| xx@xx.xx | |
| Phone | +1(800)548-4873 |
| xx@xx.xx | |
| Phone | +1(800)548-4873 |
| xx@xx.xx | |
| Phone | +1(800)548-4873 |
| xx@xx.xx | |
| Phone | +1(800)548-4873 |
| xx@xx.xx | |
| Phone | +1(800)548-4873 |
| xx@xx.xx | |
| Phone | +1(800)548-4873 |
| xx@xx.xx | |
| Phone | +1(800)548-4873 |
| xx@xx.xx | |
| Phone | +1(800)548-4873 |
| xx@xx.xx | |
| Phone | +1(800)548-4873 |
| xx@xx.xx |
Operating and Storage Conditions
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 25 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00724995182557 [Primary] |
| GS1 | 10724995182554 [Package] Contains: 00724995182557 Package: Case [20 Units] In Commercial Distribution |
FDA Product Code
| FGX | SNARE, NON-ELECTRICAL |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-04-08 |
| Device Publish Date | 2021-03-31 |
On-Brand Devices [Exacto]
| 10724995182615 | The Exacto cold snare is intended to be used without diathermic energy for the endoscopic resect |
| 10724995182561 | The Exacto cold snare is intended to be used without diathermic energy for the endoscopic resect |
| 10724995182554 | The Exacto cold snare is intended to be used without diathermic energy for the endoscopic resect |
Trademark Results [Exacto]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
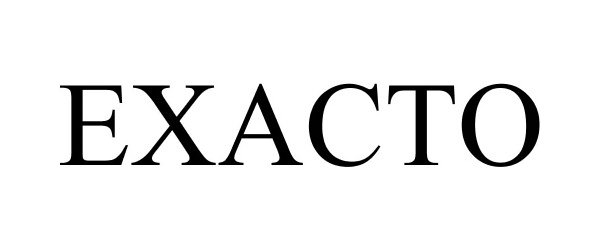 EXACTO 88092536 not registered Live/Pending |
ExactoAI Solutions LLC 2018-08-24 |
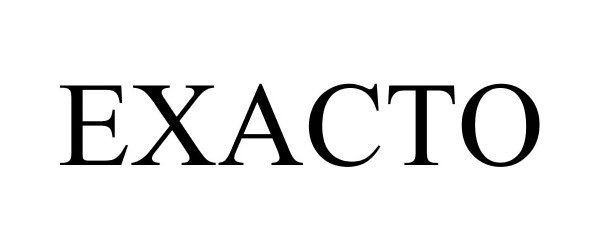 EXACTO 87533952 not registered Dead/Abandoned |
Clipper Pty Ltd 2017-07-19 |
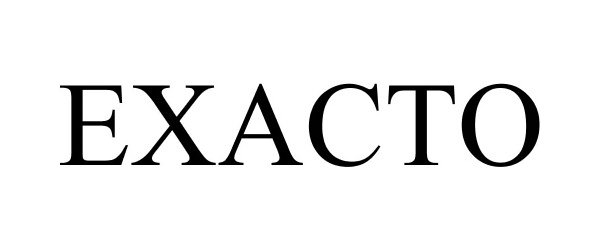 EXACTO 86743667 4991714 Live/Registered |
Exacto, Inc. 2015-09-01 |
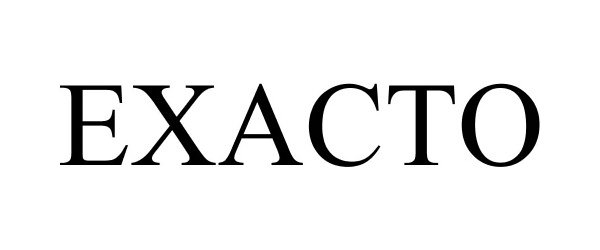 EXACTO 86696221 4901833 Live/Registered |
Durst Corporation, Inc. 2015-07-17 |
 EXACTO 85254316 4134073 Live/Registered |
U.S. Endoscopy Group, Inc. 2011-03-01 |
 EXACTO 79233594 not registered Live/Pending |
HCL TECHNOLOGIES LTD 2018-01-11 |
 EXACTO 79099268 4130334 Dead/Cancelled |
Clipper Pty Ltd 2011-06-07 |
 EXACTO 79099268 4130334 Dead/Cancelled |
Clipper Pty Ltd ACN: 122 751 642 2011-06-07 |
 EXACTO 79034124 3362722 Dead/Cancelled |
BIOSYNEX 2006-09-07 |
 EXACTO 78034056 2510640 Dead/Cancelled |
D'ANDREA MANUFACTURING CO, INC. 2000-11-06 |
 EXACTO 76413220 2807880 Dead/Cancelled |
Exacto Inc. 2002-05-28 |
 EXACTO 76230817 2633098 Dead/Cancelled |
Gloves, Inc. 2001-03-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.