Trek TKL1110
GUDID 10801741201452
Trek™ Bone Lesion Biopsy Kit, 11g x 10cm Introducer, 13g x 14.8cm Biopsy
Bard Peripheral Vascular, Inc.
Vertebral bone biopsy procedure kit, single-use| Primary Device ID | 10801741201452 |
| NIH Device Record Key | 6921c8ff-84f3-42c8-bfbf-c1729b22b24a |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Trek |
| Version Model Number | TKL1110 |
| Catalog Number | TKL1110 |
| Company DUNS | 135057938 |
| Company Name | Bard Peripheral Vascular, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Unsafe |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)321-4254 |
| medical.services@crbard.com | |
| Phone | +1(800)321-4254 |
| medical.services@crbard.com | |
| Phone | +1(800)321-4254 |
| medical.services@crbard.com | |
| Phone | +1(800)321-4254 |
| medical.services@crbard.com | |
| Phone | +1(800)321-4254 |
| medical.services@crbard.com | |
| Phone | +1(800)321-4254 |
| medical.services@crbard.com |
Device Dimensions
| Length | 14.8 Centimeter |
| Needle Gauge | 13 Gauge |
| Length | 14.8 Centimeter |
| Needle Gauge | 13 Gauge |
| Length | 14.8 Centimeter |
| Needle Gauge | 13 Gauge |
| Length | 14.8 Centimeter |
| Needle Gauge | 13 Gauge |
| Length | 14.8 Centimeter |
| Needle Gauge | 13 Gauge |
| Length | 14.8 Centimeter |
| Needle Gauge | 13 Gauge |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00801741201455 [Primary] |
| GS1 | 10801741201452 [Package] Contains: 00801741201455 Package: CA [5 Units] In Commercial Distribution |
FDA Product Code
| KNW | INSTRUMENT, BIOPSY |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-05-18 |
| Device Publish Date | 2022-05-10 |
On-Brand Devices [Trek]
| 10801741201469 | Trek™ Bone Lesion Biopsy Kit, 11g x 15cm Introducer, 13g x 19.8cm Biopsy |
| 10801741201445 | Trek™ Bone Lesion Biopsy Kit, 11g x 6cm Introducer, 13g x 10.8cm Biopsy |
| 10801741201438 | Trek™ Bone Lesion Biopsy Kit, 10g x 15cm Introducer, 12g x 19.8cm Biopsy |
| 10801741201421 | Trek™ Bone Lesion Biopsy Kit, 10g x 10cm Introducer, 12g x 14.8cm Biopsy |
| 10801741201414 | Trek™ Bone Lesion Biopsy Kit, 10g x 6cm Introducer, 12g x 10.8 cm Biopsy |
| 10801741201384 | Trek™ Bone Marrow Biopsy Kit, 11g x 15cm |
| 10801741201377 | Trek™ Bone Marrow Biopsy Kit, 11g x 10 cm |
| 00801741201356 | Trek™ Power Driver |
| 10801741201452 | Trek™ Bone Lesion Biopsy Kit, 11g x 10cm Introducer, 13g x 14.8cm Biopsy |
| 20801741201503 | Trek™ Sterile Sleeve |
Trademark Results [Trek]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TREK 98840788 not registered Live/Pending |
Ford Family Adventures, LLC 2024-11-06 |
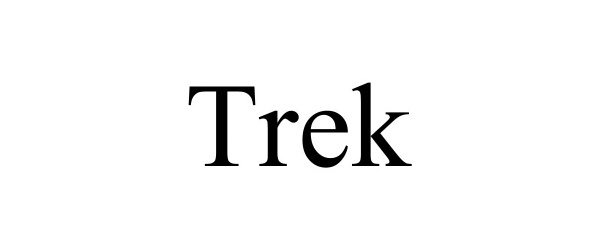 TREK 98160631 not registered Live/Pending |
Above Property, LLC 2023-09-01 |
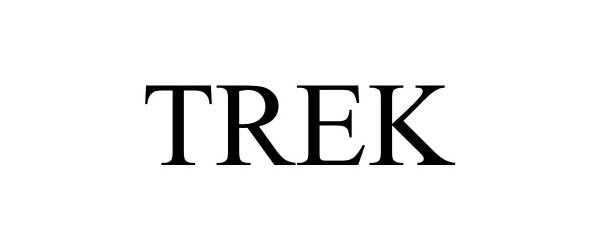 TREK 97593295 not registered Live/Pending |
Trek Bicycle Corporation 2022-09-15 |
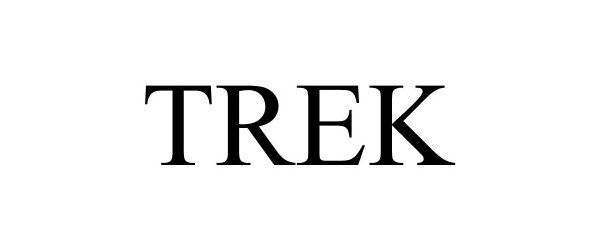 TREK 97352048 not registered Live/Pending |
Encore Coatings, LLC 2022-04-07 |
 TREK 97278572 not registered Live/Pending |
Adorama, Inc. 2022-02-22 |
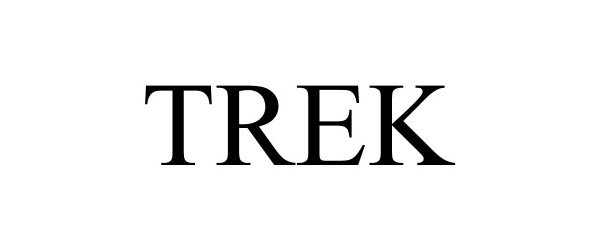 TREK 97081443 not registered Live/Pending |
Desert Leasing & Licensing LLC 2021-10-19 |
 TREK 90854879 not registered Live/Pending |
Trek Bicycle Corporation 2021-07-29 |
 TREK 90763641 not registered Live/Pending |
Trek Bicycle Corporation 2021-06-09 |
 TREK 90763580 not registered Live/Pending |
Trek Bicycle Corporation 2021-06-09 |
 TREK 90763551 not registered Live/Pending |
Trek Bicycle Corporation 2021-06-09 |
 TREK 90644221 not registered Live/Pending |
Trek, Inc. 2021-04-14 |
 TREK 90641254 not registered Live/Pending |
Advanced Energy Industries, Inc. 2021-04-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.