20/20™ S475
GUDID 10810000527585
20/20™ Double Sided Mouth Mirrors (Pkg. of 6 pcs.)
PARKELL, INC.
Dental mirror, reusable| Primary Device ID | 10810000527585 |
| NIH Device Record Key | 98cda766-62c2-41a8-980c-2a005b51b32c |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | 20/20™ |
| Version Model Number | S475 |
| Catalog Number | S475 |
| Company DUNS | 009900424 |
| Company Name | PARKELL, INC. |
| Device Count | 6 |
| DM Exempt | true |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com | |
| Phone | 6312491134 |
| customerservice@parkell.com |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
| Special Storage Condition, Specify | Between 0 and 0 *Do Not Clean Ultrasonically. Do Not use Dry Heat for sterilization. Do Not subject mirrors to temperatures above 180C. |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00810000527588 [Unit of Use] |
| GS1 | 10810000527585 [Primary] |
FDA Product Code
| EAX | Mirror, Mouth |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
[10810000527585]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2024-01-29 |
| Device Publish Date | 2019-08-30 |
On-Brand Devices [20/20™]
| 10810000527585 | 20/20™ Double Sided Mouth Mirrors (Pkg. of 6 pcs.) |
| 00810000526697 | 20/20™ Double Sided Mouth Mirrors (Pkg. of 6 pcs.) |
Trademark Results [20/20]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
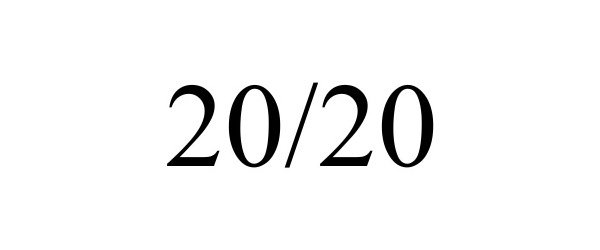 20/20 98157766 not registered Live/Pending |
Veriphy Inc 2023-08-30 |
 20/20 90196808 not registered Live/Pending |
20/20 Company 2020-09-21 |
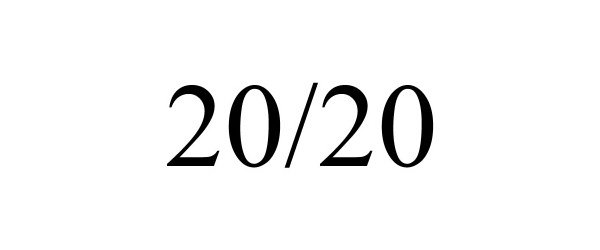 20/20 90148497 not registered Live/Pending |
RT RECOVER INNOVATIONS LTD 2020-08-31 |
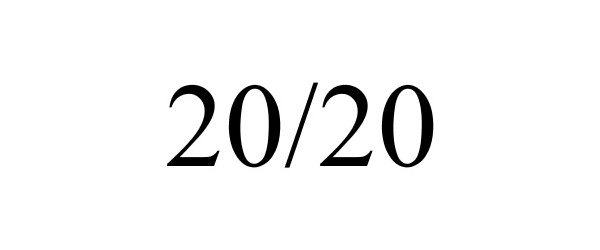 20/20 88380297 not registered Live/Pending |
20/20 Company 2019-04-10 |
 20/20 88107448 not registered Live/Pending |
Thor-lo, Inc. 2018-09-06 |
 20/20 87755296 not registered Dead/Abandoned |
20/20 IP LLC 2018-01-15 |
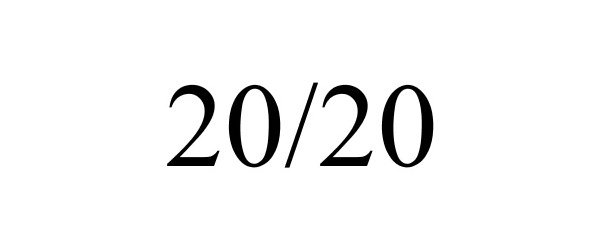 20/20 87655457 not registered Live/Pending |
Jobson Medical Information LLC 2017-10-23 |
 20/20 87522139 5406224 Live/Registered |
Jobson Medical Information LLC 2017-07-10 |
 20/20 86790405 not registered Dead/Abandoned |
Performance Support Systems, Inc. 2015-10-16 |
 20/20 86055696 4736017 Live/Registered |
ASHLAND LICENSING & INTELLECTUAL PROPERTY LLC 2013-09-04 |
 20/20 85892291 not registered Dead/Abandoned |
PRO GLASS ALLIANCE, LLC 2013-04-01 |
 20/20 85543262 4300285 Live/Registered |
Summit Electric Supply Company, Inc. 2012-02-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.