TopLock
GUDID 10810020082309
Suture
RIVERPOINT MEDICAL, LLC
Polyolefin suture, multifilament| Primary Device ID | 10810020082309 |
| NIH Device Record Key | 83532b5c-2b36-47a9-8b7a-b456baa9ba29 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | TopLock |
| Version Model Number | HS225 |
| Company DUNS | 964053560 |
| Company Name | RIVERPOINT MEDICAL, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00810020082302 [Primary] |
| GS1 | 10810020082309 [Package] Contains: 00810020082302 Package: [6 Units] In Commercial Distribution |
FDA Product Code
| GAT | Suture, Nonabsorbable, Synthetic, Polyethylene |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-03-19 |
| Device Publish Date | 2020-03-11 |
On-Brand Devices [TopLock]
| 10810020082316 | Suture |
| 10810020082309 | Suture |
| 10810020082293 | Suture |
| 10810020082286 | Suture |
Trademark Results [TopLock]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
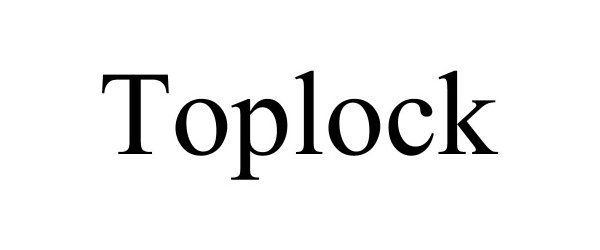 TOPLOCK 90610763 not registered Live/Pending |
Kaiping Jumei Sanitary Technology Co., Ltd. 2021-03-29 |
 TOPLOCK 86842778 5016814 Live/Registered |
TOP LOCK CORPORATION 2015-12-08 |
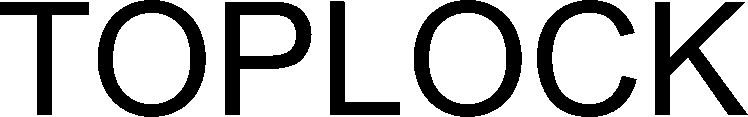 TOPLOCK 79320092 not registered Live/Pending |
Röhm GmbH 2021-05-18 |
 TOPLOCK 78398157 3488602 Dead/Cancelled |
VERMOP Salmon GmbH 2004-04-07 |
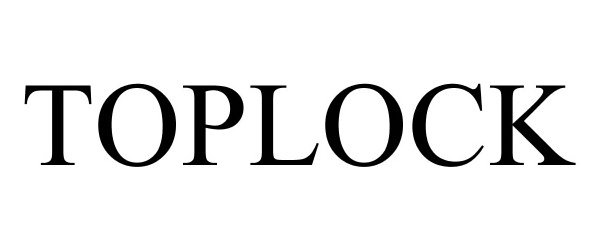 TOPLOCK 77606381 not registered Dead/Abandoned |
QTRCO, Inc. 2008-11-03 |
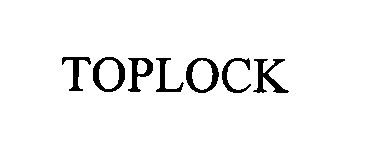 TOPLOCK 76327918 not registered Dead/Abandoned |
VERMOP SALMON GmbH 2001-10-19 |
 TOPLOCK 76327917 not registered Dead/Abandoned |
VERMOP SALMON GmbH 2001-10-19 |
 TOPLOCK 76327587 not registered Dead/Abandoned |
VERMOP SALMON GmbH 2001-10-19 |
 TOPLOCK 76327586 not registered Dead/Abandoned |
VERMOP SALMON GmbH 2001-10-19 |
 TOPLOCK 76327585 not registered Dead/Abandoned |
VERMOP SALMON GmbH 2001-10-19 |
 TOPLOCK 73734518 1581135 Dead/Cancelled |
MSC MANAGEMENT SOFTWARE CORPORATION 1988-06-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.