ABBy
GUDID 10814247021134
ABBy Panniculus Retractor - 05 Extra Large
CLINICAL INNOVATIONS, LLC
Panniculus skin-adhesive retractor, reusable| Primary Device ID | 10814247021134 |
| NIH Device Record Key | f9390e1e-98ca-4533-a612-378aa61905a1 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | ABBy |
| Version Model Number | PRSA-05XL |
| Company DUNS | 809524291 |
| Company Name | CLINICAL INNOVATIONS, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00814247021137 [Primary] |
| GS1 | 10814247021134 [Package] Contains: 00814247021137 Package: [5 Units] In Commercial Distribution |
FDA Product Code
| KGX | Tape And Bandage, Adhesive |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-04-24 |
| Device Publish Date | 2023-04-14 |
On-Brand Devices [ABBy]
| 00814247021182 | ABBy Panniculus Retractor - Eval Kit |
| 10814247021172 | ABBy Panniculus Retractor - 10 Small |
| 10814247021165 | ABBy Panniculus Retractor - 05 Small |
| 10814247021158 | ABBy Panniculus Retractor - 10 Extra Large |
| 10814247021141 | ABBy Panniculus Retractor - 10 Regular |
| 10814247021134 | ABBy Panniculus Retractor - 05 Extra Large |
| 10814247021127 | ABBy Panniculus Retractor - 05 Regular |
Trademark Results [ABBy]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
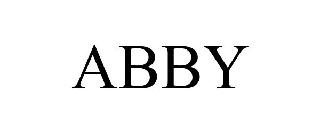 ABBY 98566152 not registered Live/Pending |
Larson, Abby 2024-05-23 |
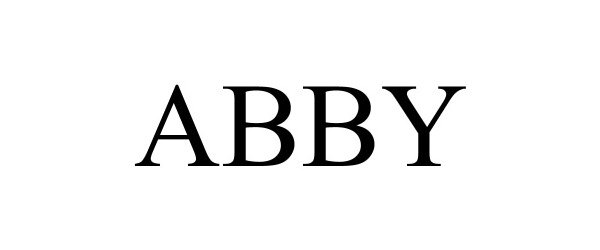 ABBY 98465938 not registered Live/Pending |
Wellspring Care Inc. 2024-03-25 |
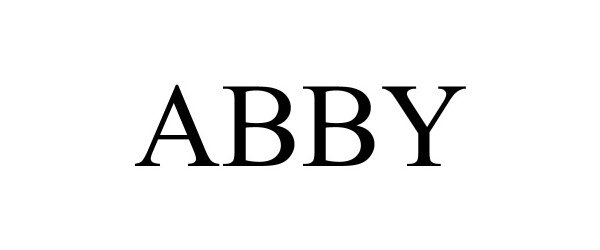 ABBY 98382591 not registered Live/Pending |
ProteinSimple Corporation 2024-01-30 |
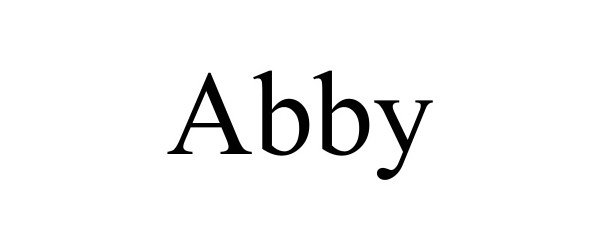 ABBY 98226110 not registered Live/Pending |
Overstock.com, Inc. 2023-10-16 |
 ABBY 97128041 not registered Live/Pending |
CL Design Studio LLC 2021-11-16 |
 ABBY 97088255 not registered Live/Pending |
CL Design Studio LLC 2021-10-22 |
 ABBY 97055196 not registered Live/Pending |
CL Design Studio LLC 2021-09-30 |
 ABBY 97055192 not registered Live/Pending |
CL Design Studio LLC 2021-09-30 |
 ABBY 97055186 not registered Live/Pending |
CL Design Studio LLC 2021-09-30 |
 ABBY 97055173 not registered Live/Pending |
CL Design Studio LLC 2021-09-30 |
 ABBY 97055152 not registered Live/Pending |
CL Design Studio LLC 2021-09-30 |
 ABBY 90902104 not registered Live/Pending |
ABB/Con-Cise Optical Group LLC 2021-08-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.