Prime Plus
GUDID 10818566016635
Earloop Procedure Mask, 7" x 3 5/8", Blue, 50/bx; 6 bxs/cs
SVS LLC
Surgical/medical face mask, single-use| Primary Device ID | 10818566016635 |
| NIH Device Record Key | 1770b372-6e22-4f0c-ae1e-fd7e080ef7c2 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Prime Plus |
| Version Model Number | 21-2415 |
| Company DUNS | 831375089 |
| Company Name | SVS LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com | |
| Phone | 1-855-531-7699 |
| udi@s2s-global.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00818566016638 [Primary] |
| GS1 | 10818566016635 [Package] Contains: 00818566016638 Package: Case [300 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K123787
FDA Product Code
| FXX | Mask, Surgical |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 6 |
| Public Version Date | 2020-05-06 |
| Device Publish Date | 2016-06-15 |
On-Brand Devices [Prime Plus]
| 10818566016642 | Earloop Procedure Mask, Yellow, 50/bx; 6bxs/cs |
| 10818566016635 | Earloop Procedure Mask, 7" x 3 5/8", Blue, 50/bx; 6 bxs/cs |
Trademark Results [Prime Plus]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PRIME PLUS 90767262 not registered Live/Pending |
Prime Buchholz LLC 2021-06-10 |
 PRIME PLUS 87019641 not registered Live/Pending |
Premier Healthcare Alliance, LP 2016-04-29 |
 PRIME PLUS 86944300 5224223 Live/Registered |
Societe des Produits Nestle S.A. 2016-03-17 |
 PRIME PLUS 78859256 3327642 Dead/Cancelled |
Allianz Life Insurance Company of NorthAmerica 2006-04-11 |
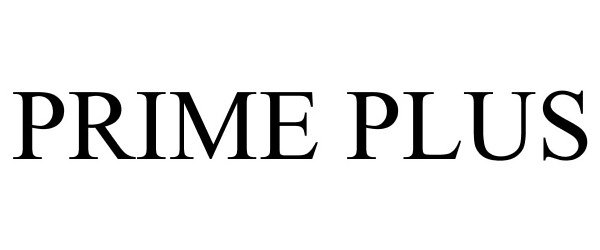 PRIME PLUS 77877641 3834216 Live/Registered |
HEXION INC. 2009-11-20 |
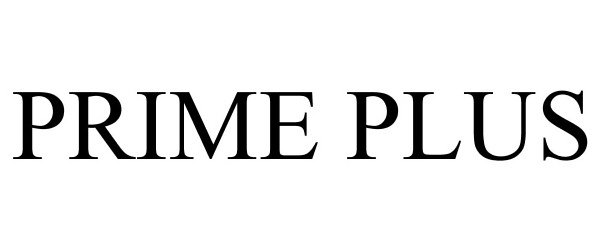 PRIME PLUS 77782424 3958531 Dead/Cancelled |
MEDICAL DEPOT, INC. 2009-07-16 |
 PRIME PLUS 74732385 not registered Dead/Abandoned |
Giblin, Karen L. 1995-09-21 |
 PRIME PLUS 74731726 2148075 Dead/Cancelled |
Giblin, Karen L. 1995-09-21 |
 PRIME PLUS 74332232 1848954 Dead/Cancelled |
AMS HEALTH SCIENCES, LLC 1992-11-16 |
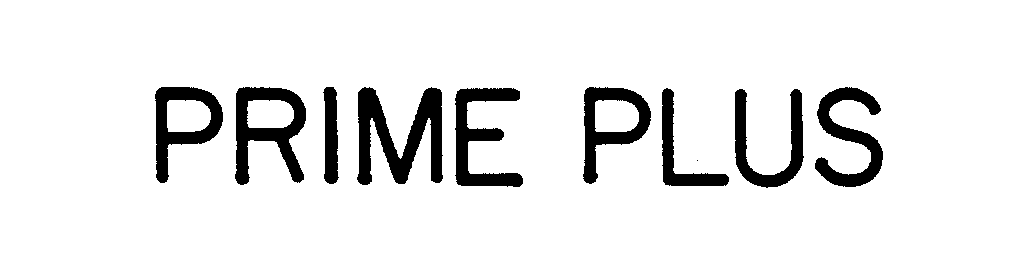 PRIME PLUS 73718223 1546026 Dead/Cancelled |
LUSTGARTEN MULTI-TECH INTERNATIONAL INC. 1988-03-22 |
 PRIME PLUS 73691715 1491321 Dead/Cancelled |
E. I. DU PONT DE NEMOURS AND COMPANY 1987-10-26 |
 PRIME PLUS 73428657 not registered Dead/Abandoned |
GIRARD BANK DELAWARE NATIONAL ASSOCIATION 1983-06-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.