Blom
GUDID 10841470103320
PULMODYNE INC
Basic tracheostomy tube, single-use| Primary Device ID | 10841470103320 |
| NIH Device Record Key | e07a33ca-a718-4e98-811e-82f9738553aa |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Blom |
| Version Model Number | 313-13-060 |
| Company DUNS | 030619483 |
| Company Name | PULMODYNE INC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 317-246-5505 |
| info@pulmodyne.com |
Device Dimensions
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00841470103323 [Primary] |
| GS1 | 10841470103320 [Package] Contains: 00841470103323 Package: [10 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K062104
FDA Product Code
| BTO | Tube, Tracheostomy (W/Wo Connector) |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2015-11-13 |
On-Brand Devices [Blom]
Trademark Results [Blom]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
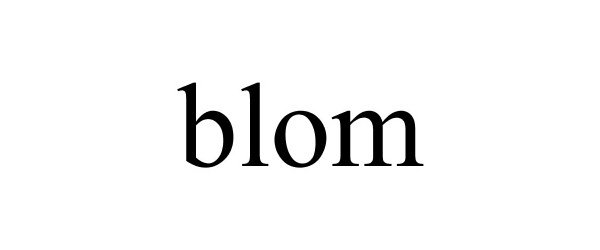 BLOM 87664164 5485901 Live/Registered |
BLOM Limited Corporation 2017-10-30 |
 BLOM 86781621 5152126 Live/Registered |
BLOM Limited 2015-10-08 |
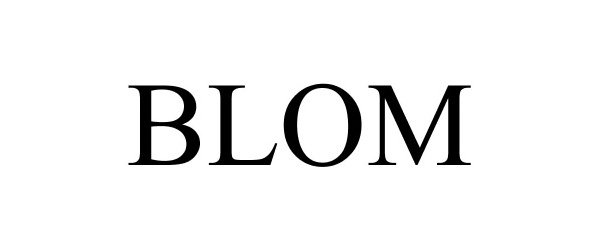 BLOM 86348617 4707669 Live/Registered |
Hansa Medical Products, Inc. 2014-07-25 |
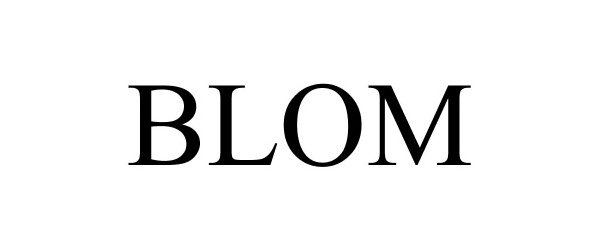 BLOM 77282240 3610558 Dead/Cancelled |
Hansa Medical Products, Inc. 2007-09-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.